��Ŀ����
һ�������£������Ϊ10L���ܱ������У�1mol A��1mol B���з�Ӧ��2A��g��+B��g���T2C��g������60s�ﵽƽ�⣬����0.6mol C������˵����ȷ���ǣ�������
| A����AŨ�ȱ仯��ʾ�ķ�Ӧ����Ϊ0.001mol?L-1?s-1 |
| B�������������䣬�����������Ϊ5L��C��ƽ��Ũ�ȱ�Ϊԭ����2�� |
| C�������������䣬������ѹǿ��������A��ת���ʼ�С |
| D���ﵽƽ��ʱ��C������ٷֺ���Ϊ0.353 |
���ݻ�ѧƽ������ʽ��ʽ����
2A��g��+B��g���T2C��g��
��ʼ����mol�� 1 1 0
�仯����mol��0.6 0.3 0.6
ƽ������mol��0.4 0.7 0.6
A����AŨ�ȱ仯��ʾ�ķ�Ӧ����=
=0.001mol?L-1?s-1����A��ȷ��
B�������������䣬�����������Ϊ5L��C��Ũ�ȱ�Ϊԭ����2��������Ӧǰ����������仯����С���ѹǿ����ƽ��������У�C��ƽ��Ũ�ȱ����ԭ����2������B����
C�������������䣬������ѹǿ��ƽ��������У�������A��ת��������C����
D���ﵽƽ��ʱ��C������ٷֺ���=
��100%=35.3%����D��ȷ��
��ѡAD��
2A��g��+B��g���T2C��g��
��ʼ����mol�� 1 1 0
�仯����mol��0.6 0.3 0.6
ƽ������mol��0.4 0.7 0.6
A����AŨ�ȱ仯��ʾ�ķ�Ӧ����=
| ||
| 60s |
B�������������䣬�����������Ϊ5L��C��Ũ�ȱ�Ϊԭ����2��������Ӧǰ����������仯����С���ѹǿ����ƽ��������У�C��ƽ��Ũ�ȱ����ԭ����2������B����
C�������������䣬������ѹǿ��ƽ��������У�������A��ת��������C����
D���ﵽƽ��ʱ��C������ٷֺ���=
| 0.6mol |
| (2-0.3)mol |
��ѡAD��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����CO��g��+2H2��g��CH3OH��g����������������и��⣺
һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����CO��g��+2H2��g��CH3OH��g����������������и��⣺ һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����CO��g��+2H2��g��?CH3OH��g���������й�˵����ȷ���ǣ�������
һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����CO��g��+2H2��g��?CH3OH��g���������й�˵����ȷ���ǣ�������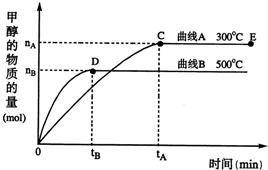 �������������ЧӦ����Դ��ȱ����������ӣ���ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ����ӣ�Ŀǰ��ҵ����һ�ַ�������CO2������ȼ�ϼ״���һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����
�������������ЧӦ����Դ��ȱ����������ӣ���ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�������˸������ձ����ӣ�Ŀǰ��ҵ����һ�ַ�������CO2������ȼ�ϼ״���һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO���� CH3OH��g��
CH3OH��g�� 2Cu+CO2
2Cu+CO2
 һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO������������������и��⣺
һ�������£������Ϊ3L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO������������������и��⣺