��Ŀ����
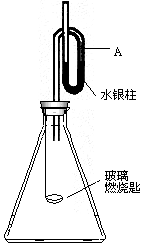
����ͼװ���װ�ã����������ѹǿ����ʱ��ע��ˮ��Һ��Ŀ̶�(�ú���)��ȡ����Ƥ�����ڲ���ȼ�ճ��м�����ۣ��þƾ��Ƶ�ȼѸ��������ƿ�в�������Ƥ������۰�����ȼ�գ�ˮ����������ܣ�������Ϩ����ã�ˮ�����������ص�ԭ�ȱ궨�Ŀ̶ȣ���Ҫ�ش��������⣺
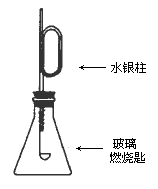
(1)ˮ�����������˵����________��
(2)���δȼ��ʱ�����Ϩ���ˣ�˵��________��
(3)����ˮ��������ֻص�ԭ�ȱ궨�Ŀ̶ȣ��ɵõ��Ľ�����________��
(4)���ݷ�Ӧ����ʽS��O2��SO2���������ۿ��Ƶ���(��֤��)________��
������
|
����(1)�ٸ÷�Ӧ�Ƿ��ȷ�Ӧ���������������� ����(2)ƿ�������Ѻľ� ����(3)��ͬ�¡�ͬѹ�£���Ӧ���ĵ����������ɵ�SO2�����ͬ����ѹǿ��ͬ�������ʵ�����ͬ ����(4)ͬ�¡�ͬѹ�£���ͬ��������庬����ͬ�ķ�����(�������ӵ�����) |

 ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�
| |||||||||||||||||||
| |||||||||||||||||||