题目内容
化学反应N2+3H2=2NH3的能量变化如下图所示,该反应的热化学方程式是

[ ]
A.N2(g)+3H2(g) === 2NH3(1) △H=2(a-b-c)kJ·mol-1
B.N2(g)+3H2(g) === 2NH3(g) △H=2(b-a)kJ·mol-1
C.N2(g)+H2(g) === NH3(1) △H=(b+c-a)kJ·mol-1
D.N2(g)+H2(g) === NH3(g) △H=(a+b)kJ·mol-1
B.N2(g)+3H2(g) === 2NH3(g) △H=2(b-a)kJ·mol-1
C.N2(g)+H2(g) === NH3(1) △H=(b+c-a)kJ·mol-1
D.N2(g)+H2(g) === NH3(g) △H=(a+b)kJ·mol-1
A

练习册系列答案
相关题目
 化学反应N2+3H2→2NH3的能量变化如图所示,该反应的热化学方程式是( )
化学反应N2+3H2→2NH3的能量变化如图所示,该反应的热化学方程式是( )| A、N2(g)+H2(g)→NH3(1)-46 kJ | B、N2(g)+H2(g)→NH3(g)-454 kJ | C、N2(g)+3 H2(g)→2 NH3(g)+92 kJ | D、N2(g)+3 H2(g)→2 NH3(1)+431.3 kJ |
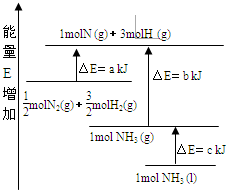 化学反应N2+3H2?2NH3的能量变化如图所示,E是正值,该反应的热化学方程式是( )
化学反应N2+3H2?2NH3的能量变化如图所示,E是正值,该反应的热化学方程式是( )| A、N2(g)+3H2(g)?2NH3(l);△H=2(a-b-c)kJ?mol-1 | ||||
| B、N2(g)+3H2(g)?2NH3(g);△H=2(b-a)kJ?mol-1 | ||||
C、
| ||||
D、
|
 (2008?重庆)化学反应N2+3H2=2NH3的能量变化如图所示,该反应的热化学方程式是( )
(2008?重庆)化学反应N2+3H2=2NH3的能量变化如图所示,该反应的热化学方程式是( )