��Ŀ����
����ʯ��������Ṥҵ��β��(��NO��NO2)��Ӧ�����ܾ���β�������ܻ��Ӧ�ù㷺��
Ca(NO2)2���䲿�ֹ����������£�
Ca(NO2)2���䲿�ֹ����������£�

(1)һ�������£�NO��NO2�������з�Ӧ��NO(g)+NO2(g) N2O3(g)����ƽ�ⳣ������ʽΪK=_______��
N2O3(g)����ƽ�ⳣ������ʽΪK=_______��
(2)���������в�����--Һ�����Ӵ�����(β�������������룬ʯ�����������������)����Ŀ����____________;������ѭ��ʹ�ã���������Ҫ�ɷ���______(�ѧʽ)��
(3)�ù��������NO��NO2���ʵ���֮�Ƚӽ�1:1����n(NO)��n(NO2)>1:1����ᵼ��__________________;��n(NO)��n(NO2)<1:1����ᵼ��__________________��
(4)��������Һ�豣�������ԣ���������Һ��Ca(NO2)2�ᷢ���ֽ⣬����֮һ��NO���䷴Ӧ�����ӷ���ʽΪ__________________��
 N2O3(g)����ƽ�ⳣ������ʽΪK=_______��
N2O3(g)����ƽ�ⳣ������ʽΪK=_______�� (2)���������в�����--Һ�����Ӵ�����(β�������������룬ʯ�����������������)����Ŀ����____________;������ѭ��ʹ�ã���������Ҫ�ɷ���______(�ѧʽ)��
(3)�ù��������NO��NO2���ʵ���֮�Ƚӽ�1:1����n(NO)��n(NO2)>1:1����ᵼ��__________________;��n(NO)��n(NO2)<1:1����ᵼ��__________________��
(4)��������Һ�豣�������ԣ���������Һ��Ca(NO2)2�ᷢ���ֽ⣬����֮һ��NO���䷴Ӧ�����ӷ���ʽΪ__________________��
(1)c(N2O3)/[c(NO)��c(NO2)]
(2)ʹβ���е�NO��NO2��������գ�Ca(OH)2
(3)�ŷ�������NO�������ߣ���ƷCa(NO2)2��Ca(NO3)2��������
(4)3NO2-+2H+=NO3-+2NO��+H2O
(2)ʹβ���е�NO��NO2��������գ�Ca(OH)2
(3)�ŷ�������NO�������ߣ���ƷCa(NO2)2��Ca(NO3)2��������
(4)3NO2-+2H+=NO3-+2NO��+H2O

��ϰ��ϵ�д�
�����Ŀ



 N2O3(g)����ƽ�ⳣ������ʽΪK��________��
N2O3(g)����ƽ�ⳣ������ʽΪK��________��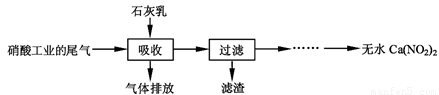
 N2O3(g)����ƽ�ⳣ������ʽΪK=
��
N2O3(g)����ƽ�ⳣ������ʽΪK=
��
 N2O3(g)����ƽ�ⳣ������ʽΪK��________��
N2O3(g)����ƽ�ⳣ������ʽΪK��________��