��Ŀ����
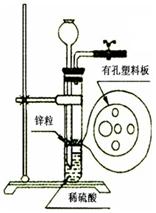
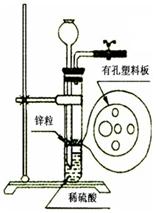
ijͬѧ��ʵ���Ҵ���ͼI��ǩ���Լ�ƿ��ȡ�����ƽ���ȼ��ʵ�飨��ͼ��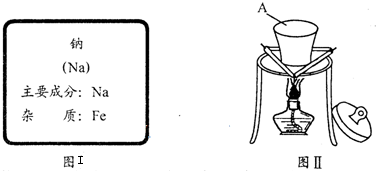
��1��װ��A������
��2��ʵ����ֻ���������ɫ�������ɣ��ӷ�Ӧ�Pʵ������²⣺�ú�ɫ���ʿ���Ϊ̼����һ����������ɵĻ�����������������
��3���Ժ�ɫ�������ʵ��������ͼ̽����
��ʵ��I����������Һ��Ŀ����
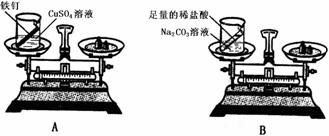
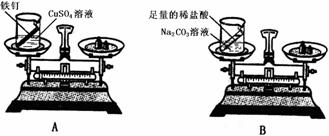
�ڽ�ͨ��ʵ�����������ܿ���ȷ����ɫ���ʵ���ɣ������Ƹ���ƣ�����ѡ�Լ���ϡ���ᡢKSCN��Һ��K3Fe��CN��6��Һ��10%H2O2��
| ʵ����� | Ԥ�ڵ���������� | ��ػ�ѧ����ʽ |
| ȡ����ʵ��������Һ������ |
|
|
��������1����������A�Ĺ����֪AΪ�������۲쵽�ĵ���ɫ����Ϊ��������ȼ�ղ���Na2O2����Ԫ����ɫ��Ӧ�Ļ�����ɫΪ��ɫ��
��2������ͼ1��֪���������к���������������������������ɫ�жϺ�ɫ���ʵ���ɿ���Ϊ����������������������
��3���ٺ�ɫ�������ʿ���Ϊ̼�����Ļ�������������Լ����Ƿ���̼��ͬʱ���������ܽ⣻
��������������������������Һ��һ�����������ӣ�����ͨ�����������ӽ����ж���ɫ������ɣ�����Һ��ɺ�ɫ��˵������Fe3O4����û�б�ɺ�ɫ��˵����ɫ����ΪFeO��д������������������ӷ�Ӧ�����ӷ���ʽ��
��2������ͼ1��֪���������к���������������������������ɫ�жϺ�ɫ���ʵ���ɿ���Ϊ����������������������
��3���ٺ�ɫ�������ʿ���Ϊ̼�����Ļ�������������Լ����Ƿ���̼��ͬʱ���������ܽ⣻
��������������������������Һ��һ�����������ӣ�����ͨ�����������ӽ����ж���ɫ������ɣ�����Һ��ɺ�ɫ��˵������Fe3O4����û�б�ɺ�ɫ��˵����ɫ����ΪFeO��д������������������ӷ�Ӧ�����ӷ���ʽ��
�����1������ͼʾ����A�Ĺ����֪��A����Ϊ�������ڼ�������������������Ӧ���ɵ���ɫ��Na2O2����Ԫ����ɫ��Ӧ�Ļ�����ɫ����ֱ�ӹ۲쵽���������ɫΪ��ɫ��
�ʴ�Ϊ��������Na2O2�� Na�����ƣ���
��2��ʵ����ֻ���������ɫ�������ɣ�����ͼ1��֪���������л���������������������Ӧ���ɵĺ�ɫ������Fe3O4��FeO���ʸ������������Fe3O4��FeO��
�ʴ�Ϊ��Fe3O4��FeO��
��3�������ں�ɫ�������ʿ���Ϊ̼�����������̼���ʲ��������ᣬ�����������ܹ���Һ���ᣬ���Լ�������Ŀ���Ǽ����ɫ�����������Ƿ���̼��ͬʱ�ܽ������
�ʴ�Ϊ�������ɫ�����������Ƿ���̼��ͬʱ�ܽ������
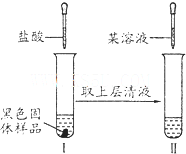
��ȡ����ʵ��������Һ������������KSCN��Һ�������Һ�Ժ�ɫ�����ɫ����ΪFe3O4�������Һû�б�ɺ�ɫ��˵����ɫ����ΪFeO����Ӧ�����ӷ���ʽΪ��Fe3++3SCN-�TFe��SCN��3��
�ʴ�Ϊ��������KSCN��Һ�������Һ�Ժ�ɫ�����ɫ����ΪFe3O4����֮��ΪFeO��Fe3++3SCN-�TFe��SCN��3��
�ʴ�Ϊ��������Na2O2�� Na�����ƣ���
��2��ʵ����ֻ���������ɫ�������ɣ�����ͼ1��֪���������л���������������������Ӧ���ɵĺ�ɫ������Fe3O4��FeO���ʸ������������Fe3O4��FeO��
�ʴ�Ϊ��Fe3O4��FeO��
��3�������ں�ɫ�������ʿ���Ϊ̼�����������̼���ʲ��������ᣬ�����������ܹ���Һ���ᣬ���Լ�������Ŀ���Ǽ����ɫ�����������Ƿ���̼��ͬʱ�ܽ������
�ʴ�Ϊ�������ɫ�����������Ƿ���̼��ͬʱ�ܽ������
��ȡ����ʵ��������Һ������������KSCN��Һ�������Һ�Ժ�ɫ�����ɫ����ΪFe3O4�������Һû�б�ɺ�ɫ��˵����ɫ����ΪFeO����Ӧ�����ӷ���ʽΪ��Fe3++3SCN-�TFe��SCN��3��
�ʴ�Ϊ��������KSCN��Һ�������Һ�Ժ�ɫ�����ɫ����ΪFe3O4����֮��ΪFeO��Fe3++3SCN-�TFe��SCN��3��
���������⿼���˽����Ƽ��仯��������ʡ�������ѧ�����Ĺ��켰���ƣ���Ŀ�Ѷ��еȣ�Ҫ��ѧ�����ս����Ƽ��仯������еĻ�ѧ���ʣ���ȷ���������Ӻ��������ӵķ�����

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 ��2011?����ģ�⣩����ѧ�뼼����ģ��
��2011?����ģ�⣩����ѧ�뼼����ģ��