��Ŀ����
��η�ֹ������ʴ�ǹ�ҵ���о����ص����ݣ�Ϊ�о�����ʴ��Ӱ�����أ�ijͬѧ��������̽��ʵ�飺| ��� | ���� | ʵ������ |
| 1 | �����½���˿���ڸ��������һ���� | �������˿������Ȼ���� |
| 2 | �����½���˿���ڳ�ʪ������һСʱ | ��˿������Ȼ���� |
| 3 | �����½���˿���ڳ�ʪ�Ŀ�����һ���� | ��˿�����ѱ�ûҰ� |
| 4 | ����ʪ����˿���ڳ��µ���������һСʱ | ��˿�������ԻҰ� |
| 5 | ����ʪ����˿���ڸ��ڳ��µ���������һСʱ | ��˿�����ѱ�ûҰ� |
| 6 | �������Ȼ�����Һ����˿���ڸ��ڳ��µ���������һСʱ | ��˿����Ұ��̶ȱ�ʵ��5���� |
��1������ʵ���з����˵绯ѧ��ʴ���ǣ���ʵ����ţ�
��2���ɸ�ʵ���֪������Ӱ������ʴ���ʵ�������
��3��Ϊ��ֹ������ʴ����ҵ���ձ���õķ�����
��4����֪��˿��Ʒ����Ϊ3.5g����ʵ����δ��ʴ������������Ϊ80%����δ��ʴ��������ϡ���ᷴӦ�������������Ϊ
��������1����˿���淢���仯��˵�������˵绯ѧ��ʴ����˿�����绯ѧ��ʴʱ������������������ʧ���ӷ���������Ӧ��
��2�����⼸��ʵ����жԱȵó�Ӱ�����أ�
��3��Ϊ��ֹ�����⣬���Բ��õ绯ѧ�����������������ı�����ṹ�ȣ�
��4��������Ʒ��δ��ʴ�������������������ᷢ����Ӧ��Fe+H2SO4=FeSO4+H2�������ݷ���ʽ�������������������
��2�����⼸��ʵ����жԱȵó�Ӱ�����أ�
��3��Ϊ��ֹ�����⣬���Բ��õ绯ѧ�����������������ı�����ṹ�ȣ�
��4��������Ʒ��δ��ʴ�������������������ᷢ����Ӧ��Fe+H2SO4=FeSO4+H2�������ݷ���ʽ�������������������
����⣺��1��3��4��5��6����˿���淢���˱仯��˵����˿�����˵绯ѧ��ʴ���ʴ�Ϊ��3��4��5��6��
��2��4��5��6ʵ��˵���¶�Ӱ�����ĸ�ʴ��1��2��3ʵ��˵��ʪ��Ӱ�����ĸ�ʴ��6ʵ��˵������ʵĴ���Ӱ�����ĸ�ʴ������Ӱ������ʴ�������У��¶ȡ�ʪ�Ⱥ͵���ʵĴ��ڣ�
�ʴ�Ϊ���¶ȩpʪ�ȩp����ʵĴ��ڣ�
��3��Ϊ���������������õķ����У���Ʃpˢ��ȱ��渲�Ƿ����������������������ȣ�
�ʴ�Ϊ����Ʃpˢ��ȱ��渲�Ƿ����������������������ȣ�
��4��������Ʒ��δ��ʴ����������=3.5g��80%=2.8g��
�����������������V����
Fe+H2SO4=FeSO4+H2��
56g 22.4L
2.8g V
V=
=1.12L��
�ʴ�Ϊ��1.12L��
��2��4��5��6ʵ��˵���¶�Ӱ�����ĸ�ʴ��1��2��3ʵ��˵��ʪ��Ӱ�����ĸ�ʴ��6ʵ��˵������ʵĴ���Ӱ�����ĸ�ʴ������Ӱ������ʴ�������У��¶ȡ�ʪ�Ⱥ͵���ʵĴ��ڣ�
�ʴ�Ϊ���¶ȩpʪ�ȩp����ʵĴ��ڣ�
��3��Ϊ���������������õķ����У���Ʃpˢ��ȱ��渲�Ƿ����������������������ȣ�
�ʴ�Ϊ����Ʃpˢ��ȱ��渲�Ƿ����������������������ȣ�
��4��������Ʒ��δ��ʴ����������=3.5g��80%=2.8g��
�����������������V����
Fe+H2SO4=FeSO4+H2��
56g 22.4L
2.8g V
V=
| 22.4L��2.8g |
| 56g |
�ʴ�Ϊ��1.12L��
���������⿼���˽����ĸ�ʴ����������öԱ�ʵ�����Ӱ������ʴ�����أ��ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
��η�ֹ������ʴ�ǹ�ҵ���о����ص����ݣ�Ϊ�о�����ʴ��Ӱ�����أ�ijͬѧ��������̽��ʵ�飺
�ش��������⣺
��1������ʵ���з����˵绯ѧ��ʴ���ǣ���ʵ����ţ� ���ڵ绯ѧ��ʴ�У�������Ӧ�� ��������Ӧ�� ��
��2���ɸ�ʵ���֪������Ӱ������ʴ���ʵ������� ��
��3��Ϊ��ֹ������ʴ����ҵ���ձ���õķ����� �������ַ�������
| ��� | ���� | ʵ������ |
| 1 | �����½���˿���ڸ��������һ���� | �������˿������Ȼ���� |
| 2 | �����½���˿���ڳ�ʪ������һСʱ | ��˿������Ȼ���� |
| 3 | �����½���˿���ڳ�ʪ�Ŀ�����һ���� | ��˿�����ѱ�ûҰ� |
| 4 | ����ʪ����˿���ڳ��µ���������һСʱ | ��˿�������ԻҰ� |
| 5 | ����ʪ����˿���ڸ��ڳ��µ���������һСʱ | ��˿�����ѱ�ûҰ� |
| 6 | �������Ȼ�����Һ����˿���ڸ��ڳ��µ���������һСʱ | ��˿����Ұ��̶ȱ�ʵ��5���� |
��1������ʵ���з����˵绯ѧ��ʴ���ǣ���ʵ����ţ�
��2���ɸ�ʵ���֪������Ӱ������ʴ���ʵ�������
��3��Ϊ��ֹ������ʴ����ҵ���ձ���õķ�����
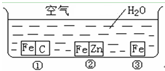 ��η�ֹ������ʴ�ǹ�ҵ���о����ص����ݣ�Ϊ�о�������ʴ��ijͬѧ����̽��ʵ�飬����ͼ��ʾ�������ڢ١��ڡ������ֲ�ͬ�Ļ����У���ش�
��η�ֹ������ʴ�ǹ�ҵ���о����ص����ݣ�Ϊ�о�������ʴ��ijͬѧ����̽��ʵ�飬����ͼ��ʾ�������ڢ١��ڡ������ֲ�ͬ�Ļ����У���ش�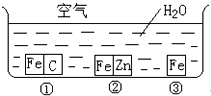 ��η�ֹ������ʴ�ǹ�ҵ���о����ص����ݣ�Ϊ�о�������ʴ��ijͬѧ����̽��ʵ�飬��ͼ��ʾ�������ڢ١��ڡ������ֲ�ͬ�Ļ����У���ش�
��η�ֹ������ʴ�ǹ�ҵ���о����ص����ݣ�Ϊ�о�������ʴ��ijͬѧ����̽��ʵ�飬��ͼ��ʾ�������ڢ١��ڡ������ֲ�ͬ�Ļ����У���ش�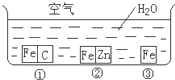 ��η�ֹ������ʴ�ǹ�ҵ���о����ص����ݣ�Ϊ�о�������ʴ��ijͬѧ����̽��ʵ�飬��ͼ��ʾ�������ڢ١��ڡ������ֲ�ͬ�Ļ����У���ش�
��η�ֹ������ʴ�ǹ�ҵ���о����ص����ݣ�Ϊ�о�������ʴ��ijͬѧ����̽��ʵ�飬��ͼ��ʾ�������ڢ١��ڡ������ֲ�ͬ�Ļ����У���ش�