��Ŀ����
��10�֣�ʵ������Ҫ0.10 mol/L NaOH��Һ475mL��0.40 mol/L��������Һ500 mL��������������Һ����������ش��������⣺

��1����ͼ��ʾ��������������Һ�϶�����Ҫ���� ������ţ�������������Һ�����õ��IJ��������� �����������ƣ���
��2�����dz������ƹ��̼���Ϊ���¸����裺
��ȷ�IJ���˳��Ӧ�� �����������ţ���
��3�������ʵ����������ƽ��ȡNaOH������Ϊ g����ʵ����������������ȷ��������ʱ�����ӿ̶��ߣ�����������ҺŨ�� 0.lmol/L������¡��������ڡ���С�ڡ�����ͬ��������Һʱ������ƿ��������������ˮ����������ҺŨ�� 0.lmol��L��
��4�����ݼ����֪��������������A 98%���ܶ�Ϊ1184g/cm3��Ũ��������Ϊ____mL������һλС������

��1����ͼ��ʾ��������������Һ�϶�����Ҫ���� ������ţ�������������Һ�����õ��IJ��������� �����������ƣ���
��2�����dz������ƹ��̼���Ϊ���¸����裺
| A����ȴ | B������ | C��ϴ�� | D������ E���ܽ� F��ҡ�ȡ� G����Һ |
��3�������ʵ����������ƽ��ȡNaOH������Ϊ g����ʵ����������������ȷ��������ʱ�����ӿ̶��ߣ�����������ҺŨ�� 0.lmol/L������¡��������ڡ���С�ڡ�����ͬ��������Һʱ������ƿ��������������ˮ����������ҺŨ�� 0.lmol��L��
��4�����ݼ����֪��������������A 98%���ܶ�Ϊ1184g/cm3��Ũ��������Ϊ____mL������һλС������

��

��ϰ��ϵ�д�
�����Ŀ
 __________________________________________________
__________________________________________________ ______________________��
______________________�� 1)����Һ����ȡ20.00 mL FeSO4��Һ������ƿ�У���0.10 mol��L��1������KMnO4��Һ�����յ㣬��ȥKMnO4��Һ20.00 mL������������MnԪ��ȫ���ʣ�2�ۣ��ζ���Ӧ�����ӷ���ʽΪ______ __���ݴ˿ɲ��FeSO4��Һ�����ʵ���Ũ��Ϊ______ __mol��L��1��
1)����Һ����ȡ20.00 mL FeSO4��Һ������ƿ�У���0.10 mol��L��1������KMnO4��Һ�����յ㣬��ȥKMnO4��Һ20.00 mL������������MnԪ��ȫ���ʣ�2�ۣ��ζ���Ӧ�����ӷ���ʽΪ______ __���ݴ˿ɲ��FeSO4��Һ�����ʵ���Ũ��Ϊ______ __mol��L��1��

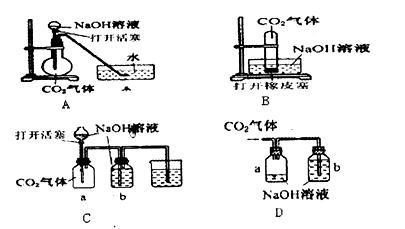
 ��ת�Ƶķ������Ŀ��
��ת�Ƶķ������Ŀ�� ѧ��Ϊ����˫ˮ�ⷴӦ���䷴Ӧ����ʽΪ2Fe3++3SO32-+6H2O=2Fe(OH)3(����)+3H2SO3��
ѧ��Ϊ����˫ˮ�ⷴӦ���䷴Ӧ����ʽΪ2Fe3++3SO32-+6H2O=2Fe(OH)3(����)+3H2SO3�� Ӧѡ�õ��Լ��� ��
Ӧѡ�õ��Լ��� ��
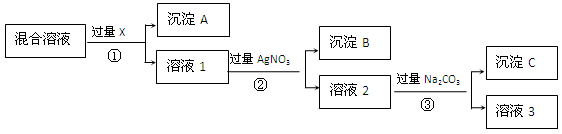
 Cl��Һ������CaCl2����������Na2CO3������
Cl��Һ������CaCl2����������Na2CO3������