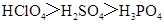��Ŀ����
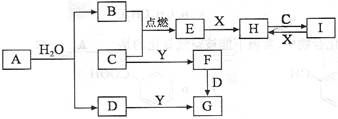
A��B��C��D�����ֳ������ʣ����ӦԪ�ص�ԭ������������������B��D���ڳ������������Ϊ���������J��һ�ֺ�ɫ���壬I��Ũ��Һ���л�ԭ�ԣ�A��J����������֮�������µ�ת����ϵ�����ַ�Ӧ����ʡ�ԣ���

��1��BԪ�غ�CԪ�صļ����Ӱ뾶��С��ϵ�� �������ӷ��ű�ʾ����
��2������Ԫ����C�γɵĻ�����NC3����ˮ�в���ʹ��ɫʯ����ֽ���������壬д���÷�Ӧ�Ļ�ѧ����ʽ ��
��3����E�ı�����Һ�����ˮ���γ���Һ�壬�ٽ���Һ��װ��U�ܣ�����U�ܵ����˲���缫����ֱͨ���磬�������˿ɹ۲쵽�������� ��
��4��������J�����ữ��H2O2����Һ�У�J�ܽ���������+2�����ӣ��÷�Ӧ�����ӷ���ʽ��
��
��5������0��1 mol G����Һ�еμ�5 mol/L ��������Һ���õ�����3��9 g �������������������Ϊ ��

��1��BԪ�غ�CԪ�صļ����Ӱ뾶��С��ϵ�� �������ӷ��ű�ʾ����
��2������Ԫ����C�γɵĻ�����NC3����ˮ�в���ʹ��ɫʯ����ֽ���������壬д���÷�Ӧ�Ļ�ѧ����ʽ ��
��3����E�ı�����Һ�����ˮ���γ���Һ�壬�ٽ���Һ��װ��U�ܣ�����U�ܵ����˲���缫����ֱͨ���磬�������˿ɹ۲쵽�������� ��
��4��������J�����ữ��H2O2����Һ�У�J�ܽ���������+2�����ӣ��÷�Ӧ�����ӷ���ʽ��
��
��5������0��1 mol G����Һ�еμ�5 mol/L ��������Һ���õ�����3��9 g �������������������Ϊ ��
��1��r��Al3+�� < r��Cl?������Al3+ < Cl?����2��NCl3+ 3H2O = NH3��+ 3HClO��3�����ɫ��dz������Cl2���ɿɸ�1�֣���4��MnO2 + H2O2 + 2H+ = Mn2+ + O2�� + 2H2O��5��10 mL��50 mL
���������I��Ũ��Һ���л�ԭ�ԣ�ӦΪHCl��J��һ�ֺ�ɫ���壬����Ũ���ᷴӦ��ӦΪMnO2�����߷�Ӧ����CΪCl2��DΪ��������������ˮ������Ӧ��ӦΪFe����EΪFeCl3��AΪH2��FΪFe3O4��B����Fe3O4�ڸ����·�Ӧ��������NaOH��Һ��Ӧ����BΪAl��GΪNaAlO2��HΪAl2O3����1�������Ϸ�����֪BԪ�صļ�����ΪAl3+��CΪCl����Al3+���������Ӳ㣬��Cl�����������Ӳ㣬��r��Al3+��< r��Cl?����2����ɫʯ����ֽ���������弴Ϊ������CΪCl2������ԭ�����غ���ƽ��Ӧ����ʽ��NCl3+ 3H2O = NH3��+ 3HclO��3��EΪFeCl3��ʵ���ҿ��ý�����FeCl3��Һ��μ����ˮ�ķ����Ʊ������������壬������е�Ӿ���������Ӵ�����ɣ���ӵ�Դ�������Դ�����ƶ�����������ɫ���4��JΪMnO2�����������֪��Ӧ�����ӷ���ʽΪMnO2+H2O2+2H+�TMn2++O2��+2H2O��5��GΪNaAlO2��������������ʱ��������ƫ�����Ʒ�Ӧ������������������NaAl(OH)4+HCl==NaCl+H2O+Al(OH)3���������nL����
n��5mol/L
 ��
�� ��5n=1��1��n=0.01L=10mL�������������ʱ��������ƫ��������ȫ��Ӧ������������������������������������������Ӧ��ʹ�����������٣���һ��0.1molƫ��������ȫ��Ӧ��NaAl(OH)4+HCl==NaCl+H2O+Al(OH)3�� ������5mol/L��������Һ20ml����0.1mol��������������0.1mol������Ϊ7.8g���ַ�Ӧ��õ�����3.9g�����ڶ�����Ӧ��������7.8g-3.9g=3.9g����ڶ�������mL���3HCl+Al(OH)3=AlCl3+3H2O��
��5n=1��1��n=0.01L=10mL�������������ʱ��������ƫ��������ȫ��Ӧ������������������������������������������Ӧ��ʹ�����������٣���һ��0.1molƫ��������ȫ��Ӧ��NaAl(OH)4+HCl==NaCl+H2O+Al(OH)3�� ������5mol/L��������Һ20ml����0.1mol��������������0.1mol������Ϊ7.8g���ַ�Ӧ��õ�����3.9g�����ڶ�����Ӧ��������7.8g-3.9g=3.9g����ڶ�������mL���3HCl+Al(OH)3=AlCl3+3H2O��m��5
 ��
�� ��5m=1��3��m=30mL
��5m=1��3��m=30mLһ��������������50mL���ʼ���������������Ϊ10 mL��50 mL��

��ϰ��ϵ�д�
�����Ŀ