��Ŀ����
һ���¶��£���a�����b�����c�������������ϡ��Һ������a��b��c������=��������д��
�ٵ������ʵ���Ũ����ͬʱ��c��H+���ɴ�С��˳����______��
��ͬ���ͬ���ʵ���Ũ�ȵ������ᣬ�к�NaOH�����ɴ�С��˳����______��
�۵���c��H+����ͬʱ�����ʵ���Ũ���ɴ�С��˳��Ϊ______��
�ܵ�c��H+����ͬ�������ͬʱ��ͬʱ������״���������ܶ���ͬ��п����������ͬ�������������ͬ״��������ʼʱ��Ӧ���ʵĴ�С��ϵ��______��
�ݽ�c��H+����ͬ�����������ˮϡ����ԭ����10����c��H+���ɴ�С��˳��Ϊ_______��
�ٵ������ʵ���Ũ����ͬʱ��c��H+���ɴ�С��˳����______��
��ͬ���ͬ���ʵ���Ũ�ȵ������ᣬ�к�NaOH�����ɴ�С��˳����______��
�۵���c��H+����ͬʱ�����ʵ���Ũ���ɴ�С��˳��Ϊ______��
�ܵ�c��H+����ͬ�������ͬʱ��ͬʱ������״���������ܶ���ͬ��п����������ͬ�������������ͬ״��������ʼʱ��Ӧ���ʵĴ�С��ϵ��______��
�ݽ�c��H+����ͬ�����������ˮϡ����ԭ����10����c��H+���ɴ�С��˳��Ϊ_______��
���Ȼ����������ǿ����ʣ���ˮ����ȫ���룬������һԪ�ᣬ����������c��H+��=c��HCl���������Ƕ�Ԫ�ᣬ������Һ��c��H+��=2c��H2SO4����������һԪ���ᣬ���Դ�����Һ��c��H+����c��CH3COOH�������Ե����ʵ���Ũ�ȵ������ᣬ������Ũ�ȴ�С˳����b��a��c���ʴ�Ϊ��b��a��c��
��ͬ���ͬ���ʵ���Ũ�ȵ������ᣬ������n��HCl��=n��CH3COOH��=n��H2SO4��������ʹ�����һԪ�ᣬ�����Ƕ�Ԫ�ᣬ����ʹ�����Ҫ�������Ƶ����ʵ�����ȣ�������Ҫ����������������ʹ����2�����к�NaOH�����ɴ�С��˳����b��a=c���ʴ�Ϊb��a=c��
���Ȼ����������ǿ����ʣ�������һԪ�ᣬ�����Ƕ�Ԫ�ᣬ�����������������һԪ�ᣬ����������������Ũ�������Ũ����ȣ�������������Ũ���������Ũ�ȵ�2����������������Ũ��С�ڴ����Ũ�ȣ��������������Ũ�����ʱ��
���ʵ���Ũ���ɴ�С��˳��Ϊc��a��b���ʴ�Ϊ��c��a��b��
����п��Ӧʱ�����⣨������������������Ũ�ȳ����ȣ�������Ũ��Խ��Ӧ����Խ��c��H+����ͬ�������ͬʱ��ͬʱ������״���������ܶ���ͬ��п����ʼʱ��Ӧ���ʵĴ�С��ϵ��a=b=c���ʴ�Ϊa=b=c��
��ǿ����Һϡ��10����������Ũ�ȱ�Ϊԭ����
��������Һϡ��10����������Ũ�ȱ�Ϊ����ԭ����
�������������ǿ�ᣬ���������ᣬ����c��H+���ɴ�С��˳��Ϊc��a=b���ʴ�Ϊc��a=b��
��ͬ���ͬ���ʵ���Ũ�ȵ������ᣬ������n��HCl��=n��CH3COOH��=n��H2SO4��������ʹ�����һԪ�ᣬ�����Ƕ�Ԫ�ᣬ����ʹ�����Ҫ�������Ƶ����ʵ�����ȣ�������Ҫ����������������ʹ����2�����к�NaOH�����ɴ�С��˳����b��a=c���ʴ�Ϊb��a=c��
���Ȼ����������ǿ����ʣ�������һԪ�ᣬ�����Ƕ�Ԫ�ᣬ�����������������һԪ�ᣬ����������������Ũ�������Ũ����ȣ�������������Ũ���������Ũ�ȵ�2����������������Ũ��С�ڴ����Ũ�ȣ��������������Ũ�����ʱ��
���ʵ���Ũ���ɴ�С��˳��Ϊc��a��b���ʴ�Ϊ��c��a��b��
����п��Ӧʱ�����⣨������������������Ũ�ȳ����ȣ�������Ũ��Խ��Ӧ����Խ��c��H+����ͬ�������ͬʱ��ͬʱ������״���������ܶ���ͬ��п����ʼʱ��Ӧ���ʵĴ�С��ϵ��a=b=c���ʴ�Ϊa=b=c��
��ǿ����Һϡ��10����������Ũ�ȱ�Ϊԭ����
| 1 |
| 10 |
| 1 |
| 10 |

��ϰ��ϵ�д�
 �ظ���ʦ�㲦ϵ�д�
�ظ���ʦ�㲦ϵ�д�
�����Ŀ




 cE��g����dX��g������2 L�ܱ������У�����4 mol D��5 mol Y���д��������£�2 min��Ӧ�ﵽƽ�⣬���ƽ��ʱ�����ڵ�ѹǿ�ȷ�Ӧǰ������1��18����ǰ2 min����E��ʾ��ƽ����Ӧ����Ϊ______mol��L��1��min-1��ƽ��ʱD��Ũ��Ϊ________mol��L��1��
cE��g����dX��g������2 L�ܱ������У�����4 mol D��5 mol Y���д��������£�2 min��Ӧ�ﵽƽ�⣬���ƽ��ʱ�����ڵ�ѹǿ�ȷ�Ӧǰ������1��18����ǰ2 min����E��ʾ��ƽ����Ӧ����Ϊ______mol��L��1��min-1��ƽ��ʱD��Ũ��Ϊ________mol��L��1��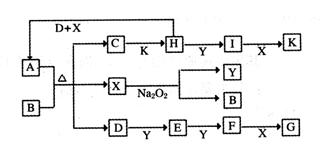
 cE��g����dX��g������2 L�ܱ������У�����4 mol
D��5 mol Y���д��������£�2 min��Ӧ�ﵽƽ�⣬���ƽ��ʱ�����ڵ�ѹǿ�ȷ�Ӧǰ������1��18����ǰ2 min����E��ʾ��ƽ����Ӧ����Ϊ______mol��L��1��min-1��ƽ��ʱD��Ũ��Ϊ________mol��L��1��
cE��g����dX��g������2 L�ܱ������У�����4 mol
D��5 mol Y���д��������£�2 min��Ӧ�ﵽƽ�⣬���ƽ��ʱ�����ڵ�ѹǿ�ȷ�Ӧǰ������1��18����ǰ2 min����E��ʾ��ƽ����Ӧ����Ϊ______mol��L��1��min-1��ƽ��ʱD��Ũ��Ϊ________mol��L��1��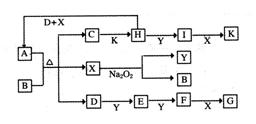
 cE��g����dX��g������2 L�ܱ������У�����4 mol D��5
mol Y���д��������£�2 min��Ӧ�ﵽƽ�⣬���ƽ��ʱ�����ڵ�ѹǿ�ȷ�Ӧǰ������1��18����ǰ2 min����E��ʾ��ƽ����Ӧ����Ϊ______mol��L��1��min-1��ƽ��ʱD��Ũ��Ϊ________mol��L��1��
cE��g����dX��g������2 L�ܱ������У�����4 mol D��5
mol Y���д��������£�2 min��Ӧ�ﵽƽ�⣬���ƽ��ʱ�����ڵ�ѹǿ�ȷ�Ӧǰ������1��18����ǰ2 min����E��ʾ��ƽ����Ӧ����Ϊ______mol��L��1��min-1��ƽ��ʱD��Ũ��Ϊ________mol��L��1��