��Ŀ����
���и���Һ�У��йسɷֵ����ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�������
| A��10mL0.5mol/LCH3COONa��Һ��6mLlmol/L�����ϣ�c��Cl-����c��Na+����c��CH3COO-����c��H+����c��OH-�� |
| B��0��lmol/LpHΪ4��NaHB��Һ�У�c��HB-����c��H2B����c��B2-�� |
| C�����������Һ�еμ�������������Һǡ�ó����ԣ�c��Na+����c��SO42-����c��NH4+����c��OH-��=c��H+�� |
| D��pH��ȵģ�NH4��2SO4��Һ����NH4��2Fe��SO4��2��Һ��NH4C1��Һ��c[��NH4��2SO4]��c[��NH4��2Fe��SO4��2]��c��NH4Cl�� |
A��10mL0.5mol/LCH3COONa��Һ��6mLlmol/L�����ϣ�������Ӧ���������Һ��CH3COOH��NaCl��HCl�����ʵ���Ũ��֮��Ϊ5��5��1������c��Cl-����c��Na+������Һ�����ԣ�c��H+����c��OH-�������������ᣬ�������룬����̶Ȳ�������c��Na+����c��CH3COO-����c��H+����c��CH3COO-������Ϊc��Cl-����c��Na+����c��H+����c��CH3COO-����c��OH-������A����
B��0.1mol?L-1��NaHB��ҺpHΪ4��˵��HB-Ϊ���������HB-����̶ȴ�����ˮ��̶ȣ���������B2-��ˮ������H2B������
c��B2-����c��H2B����������ˮ��̶Ȳ���c��HB-���������c��HB-����c��B2-����c��H2B������B����
C�����������Һ�еμ�������������Һǡ�ó����ԣ�笠�������ʣ�࣬��ҺΪ�����ơ�����李���ˮ�Ļ����Һ��������������Ƶ����ʵ�������������泥�����c��Na+����c��SO2-4�������������غ㣬n��SO42-��=n��NH4+��+n��NH3?H2O��������c��SO2-4����c��NH+4������c��Na+����c��SO2-4����c��NH+4����c��OH-��=c��H+������C��ȷ��
D��pH��ȵģ�NH4��2SO4��Һ����NH4��2Fe��SO4��2��Һ����NH4��2Fe��SO4��2��Һ��������������笠�����ˮ�⣬���ԣ�NH4��2Fe��SO4��2��ҺŨ�Ƚϴ�pH��ȵģ�NH4��2SO4��Һ��NH4C1��Һ��ֻ��笠�����ˮ�⣬��NH4��2SO4��NH4C1�ṩ��笠�����Ũ����ȣ�����2c[��NH4��2SO4]=c��NH4Cl������c[��NH4��2SO4]��c[��NH4��2Fe��SO4��2]��c��NH4Cl������D��ȷ��
��ѡ��CD��
B��0.1mol?L-1��NaHB��ҺpHΪ4��˵��HB-Ϊ���������HB-����̶ȴ�����ˮ��̶ȣ���������B2-��ˮ������H2B������
c��B2-����c��H2B����������ˮ��̶Ȳ���c��HB-���������c��HB-����c��B2-����c��H2B������B����
C�����������Һ�еμ�������������Һǡ�ó����ԣ�笠�������ʣ�࣬��ҺΪ�����ơ�����李���ˮ�Ļ����Һ��������������Ƶ����ʵ�������������泥�����c��Na+����c��SO2-4�������������غ㣬n��SO42-��=n��NH4+��+n��NH3?H2O��������c��SO2-4����c��NH+4������c��Na+����c��SO2-4����c��NH+4����c��OH-��=c��H+������C��ȷ��
D��pH��ȵģ�NH4��2SO4��Һ����NH4��2Fe��SO4��2��Һ����NH4��2Fe��SO4��2��Һ��������������笠�����ˮ�⣬���ԣ�NH4��2Fe��SO4��2��ҺŨ�Ƚϴ�pH��ȵģ�NH4��2SO4��Һ��NH4C1��Һ��ֻ��笠�����ˮ�⣬��NH4��2SO4��NH4C1�ṩ��笠�����Ũ����ȣ�����2c[��NH4��2SO4]=c��NH4Cl������c[��NH4��2SO4]��c[��NH4��2Fe��SO4��2]��c��NH4Cl������D��ȷ��
��ѡ��CD��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��10�֣��ڳ����£���һ��Ũ�ȵ�CH3COOH��Һ�ζ�V mLͬŨ��NaOH��Һʱ�õ��ĵζ���������ͼ��
���ⶨij��Һ��ֻ����Na+��CHCOO����H+��OH�� �������ӣ���֪������Һ����һ�ֻ��������ʡ������ϱ�����Ũ�ȵ�CH3COOH��CH3COONa�Ļ��Һ�����ԡ���������и��⣺
��1���Է�����ͼ����ʾ�ζ����̵�b��d������ܵ�������ϣ�
b��_____________________��d��____________________��
��2���ֱ�ָ����ͼa��c���������ڵ���������Ũ�ȴ�С��ϵ��
a�㣺_________________________________________________________________________
c�㣺_________________________________________________________________________
��3��ˮ�ĵ���̶�����Һ�����ܽ�ĵ�����йأ��Է�����ͼa��b��c��d�㣬ˮ�ĵ���̶�������______��
��4���й�������Һ�����е�˵������ȷ����_________
| A������Һ�����Ӽ����㣺c(Na+)>c(CH3COO��)>c(OH��)>c(H+)������Һ�����ʿ���ΪCH3COONa��NaOH |
| B������Һ�����Ӽ�����c(CH3COO��)>c(Na+)>c(H+)>c (OH��)������Һ������һ��ֻ��CH3COONa |
| C������Һ��c(Na+)=c(CH3COO��)�������Һһ�������� |
| D������Һ��c(CH3COOH)>c(Na+)������Һһ�������� |
��5������Һ���������ȵ�CH3COOH��Һ��NaOH��Һ��϶��ɣ���ǡ�ó����ԣ�����ǰc(CH3COOH)____________c(NaOH)���>����<����=������
 A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش�
A��B��C��X����ѧ��ѧ�������ʣ����ɶ�����Ԫ����ɣ�ת����ϵ��ͼ��ʾ����������²�ͬ����ش� ���÷����й����ŵ�����Ϊ
���÷����й����ŵ�����Ϊ
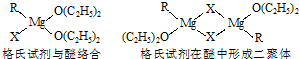
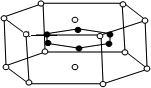

 Ϊ_______________________________________��
Ϊ_______________________________________�� �ṹ��
�ṹ��