��Ŀ����
3��ij�л���A��C4 H6O5�����㷺����������ˮ���У���һ�ֳ��õ�ʳƷ���Ӽ����û���������������ʣ�i.25��ʱ�����볣��K1=3.99��10-4��K2=5.5��10-6
ii��Imol A�������Ʋ���1.5mol H2��
ii�������к˴Ź���������ʾ��5�ֲ�ͬ��ѧ��������ԭ�ӣ���A��صķ�Ӧ�������£�
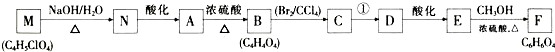
�ش��������⣺
��1��A�й����ŵ�����Ϊ�ǻ����Ȼ���B�Ľṹ��ʽΪHOOCCH=CHCOOH��C��ϵͳ����Ϊ2��3-���嶡����_��
��2��A--B�ķ�Ӧ����Ϊ��ȥ��Ӧ����Ӧ������ķ�Ӧ����Ϊ��������ˮ��Һ�����ȣ�
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
��M-N��
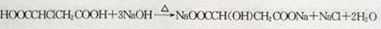 ��
����E-F��
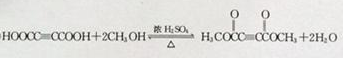 ��
����4��A�ж���ͬ���칹�壬д����������������ͬ���칹��ṹ��ʽ��HOOCCOOCH2CH2OH��
���ܷ���ˮ�⣬���������л����ˮ������֮���ܷ������۷�Ӧ��
���� A����ʽΪC4H6O5�������Ͷ�Ϊ $\frac{2��4+2-6}{2}$=2��M������������Һ���������·���ˮ���N��N�ữ��A��1mol A�������Ľ����Ʒ�Ӧ����1.5mol H2�����A�ĵ���ƽ�ⳣ������֪AΪ��Ԫ���ᣬ��A�����ǻ����Ȼ�����A�����к���һ���ǻ��Ͷ����Ȼ������B�ķ���ʽ��֪��A��Ũ���������¼��ȷ�����ȥ��Ӧ��BΪHOOCCH=CHCOOH���˴Ź������ױ���A��������5�ֲ�ͬ��ѧ��������ԭ�ӣ���A�Ľṹ��ʽΪ��HOOCCH��OH��CH2COOH��B����ӳɵ�CΪHOOCCH��Br��CH��Br��COOH������F�ķ���ʽ��֪��C����������ˮ��Һ�����������·���ˮ�ⷴӦ�õ�DΪ��D�ữ��EΪHOOCC��CCOOH��E��״�����������Ӧ��FΪCH3OOCC��CCOOCH3������A�Ľṹ��ʽ�ɷ��Ƶ�NΪNaOOCCH��OH��CH2COONa��MΪHOOCCH��Cl��CH2COOH���ݴ˽��
��� �⣺A����ʽΪC4H6O5�������Ͷ�Ϊ $\frac{2��4+2-6}{2}$=2��M������������Һ���������·���ˮ���N��N�ữ��A��1mol A�������Ľ����Ʒ�Ӧ����1.5mol H2�����A�ĵ���ƽ�ⳣ������֪AΪ��Ԫ���ᣬ��A�����ǻ����Ȼ�����A�����к���һ���ǻ��Ͷ����Ȼ������B�ķ���ʽ��֪��A��Ũ���������¼��ȷ�����ȥ��Ӧ��BΪHOOCCH=CHCOOH���˴Ź������ױ���A��������5�ֲ�ͬ��ѧ��������ԭ�ӣ���A�Ľṹ��ʽΪ��HOOCCH��OH��CH2COOH��B����ӳɵ�CΪHOOCCH��Br��CH��Br��COOH������F�ķ���ʽ��֪��C����������ˮ��Һ�����������·���ˮ�ⷴӦ�õ�DΪ��D�ữ��EΪHOOCC��CCOOH��E��״�����������Ӧ��FΪCH3OOCC��CCOOCH3������A�Ľṹ��ʽ�ɷ��Ƶ�NΪNaOOCCH��OH��CH2COONa��MΪHOOCCH��Cl��CH2COOH��
��1��A�Ľṹ��ʽΪ��HOOCCH��OH��CH2COOH��A�й����ŵ�����Ϊ�ǻ����Ȼ���BΪHOOCCH=CHCOOH��CΪHOOCCH��Br��CH��Br��COOH��C��ϵͳ����Ϊ2��3-���嶡���ᣬ
�ʴ�Ϊ���ǻ����Ȼ���HOOCCH=CHCOOH��2��3-���嶡���
��2����������ķ�����֪�ڣ�A--B�ķ�Ӧ����Ϊ��ȥ��Ӧ����Ӧ������ķ�Ӧ����Ϊ��������ˮ��Һ�����ȣ�
�ʴ�Ϊ����ȥ��Ӧ����������ˮ��Һ�����ȣ�
��3����ӦM-N�Ļ�ѧ����ʽΪ��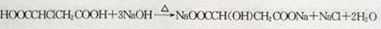 ����ӦE-F�Ļ�ѧ����ʽΪ
����ӦE-F�Ļ�ѧ����ʽΪ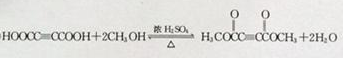 ��
��
�ʴ�Ϊ��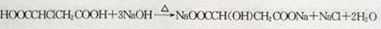 ��
��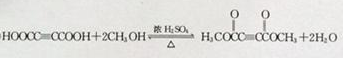 ��
��
��4��A��ͬ���칹����������������ܷ���ˮ�⣬���������л��˵������������ˮ������֮���ܷ������۷�Ӧ������������Ľṹ��ʽΪ��HOOCCOOCH2CH2OH��
�ʴ�Ϊ��HOOCCOOCH2CH2OH��
���� ���⿼���л�����ƶϣ�ȷ��A�Ľṹ��ʽ�ǹؼ����ٽ�Ϸ�Ӧ�������л������ʽ�����ƶϣ�������ѧ���ķ��������Ŀ��飬��Ҫѧ���������չ����ŵ�������ת�����Ѷ��еȣ�

 �������¿��ÿ�ʱ��ҵϵ�д�
�������¿��ÿ�ʱ��ҵϵ�д� Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�| A�� | ���Ӱ뾶��Al3+��Mg2+��Na+ | B�� | ���ȶ��ԣ�HI��HBr��HCl��HF | ||
| C�� | ���ԣ�H2CO3��H2SO4��HClO4 | D�� | ���ԣ�Al��OH��3��Mg��OH�� 2��NaOH |
| A�� | C3H8 | B�� | C3H4 | C�� | C2H2 | D�� | C3H6 |
| A�� | �� | B�� | þ | C�� | �� | D�� | �� |
| A�� | CH4$\stackrel{����}{��}$C+2H2 | |
| B�� | CH3CH=CH2+Br2$\stackrel{CCl_{4}}{��}$CH3CHBrCH2Br | |
| C�� | CH+2O2$\stackrel{��ȼ}{��}$CO2+2H2O | |
| D�� | CH4+Cl2$\stackrel{��}{��}$CH3Cl+HCl |
| A�� | ���ԣ�HCl04��HBr04��HI04 | B�� | ���ԣ�Na0H��Mg��0H��2��Al��0H��3 | ||
| C�� | �ȶ��ԣ�PH3��H2S��HCl | D�� | �ǽ����ԣ�F��0��S |
| ʵ����������� | ʵ����� | |
| A | ��SO2ͨ��Ʒ����Һ�У���ɫ����ȥ���ټ�������ɫ����Һ����Һ�ָֻ�Ϊ��ɫ | ���ɵ�������ȶ� |
| B | ��ij����Һ�м���ŨNaOH��Һ�����ȣ�����������ʹʪ��ĺ�ɫʯ����ֽ����ɫ | ԭ��Һ�к���NH+4 |
| C | ��ij��������Һ�м�������ϡH2SO4���ữ���ٵμӼ���KSCN��Һ����Һ���ɫ | ԭ������ΪFe��NO3��3 |
| D | ������Һ��ϡ�������ȣ�һ��ʱ������������Һ����ˮ�ܼ��ȣ��Թ��ڱ�δ�������� | ����һ����δˮ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |


 ��д��E��F�ķ�Ӧ���ͼӾ۷�Ӧ��
��д��E��F�ķ�Ӧ���ͼӾ۷�Ӧ��
 ��
��
 ��
�� ��CH3��2CHCOONa+Cu2O��+3H2O��
��CH3��2CHCOONa+Cu2O��+3H2O�� ��
�� ��
��