��Ŀ����
����Ŀ������̼��ƹ㷺Ӧ���������ϡ���ֽ����ѧ���ġ���ī��Ϳ�ϡ��ܷ⽺�뽺ճ������ҵ����ŨCaCl2��Һ��ͨ��NH3��CO2�������Ƶ�����̼��ơ�ijУѧ��ʵ��С�������ͼ��ʾװ�ã���ȡ�ò�Ʒ��D��װ��պϡ�������֬�ޣ�ͼ�мг�װ������ȥ��
��ѡ�õ�ҩƷ�У�
a��ʯ��ʯ��b�������Ȼ�����Һ��c��6 mol/L���d���Ȼ�泥�e����������
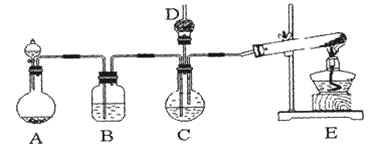
��1��A���Ʊ�����ʱ������ҩƷ�ǣ�ѡ����ĸ��ţ�______________��
��2��B��ʢ�б���̼��������Һ����������______________________________��
��3��д����ȡ�����Ļ�ѧ����ʽ__________________________________��
��4����ʵ������У���C��ͨ�����������Ⱥ�˳��ģ�Ӧ��ͨ������Ļ�ѧʽ______________��
��5������D���ڴ��Ƿ��а����ݳ��ķ�����__________________________��
��6��д��������̼��ƵĻ�ѧ����ʽ______________________________��
��7����ʵ��������а����ݳ���Ӧѡ������_____________װ�û��գ�����ţ���
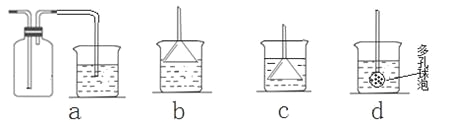
�������������Ȼ����Ʒ�к�������̼�����ơ�Ϊ�˲ⶨ�Ȼ�淋�������������ѧ��ʵ��С�������������ʵ�����̣�
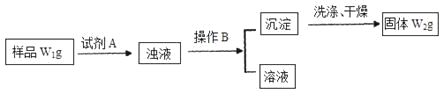
�Իش�
��1�������Լ�A�Ļ�ѧʽΪ______________________________________��
��2��B����������_______________________________________________��
��3����Ʒ���Ȼ�淋���������Ϊ___________________________________��
���𰸡�ac ��ȥCO2�е�HCl 2NH4Cl + Ca(OH)2![]() CaCl2+ 2NH3��+ 2H2O NH3 ��ʪ��ĺ�ɫʯ����ֽ����D���ڴ�������ֽ��������֤���а����ݳ�������ֽ����������֤��û�а����ݳ���������պ��Ũ����IJ���������D���ڴ������а��̣���֤���а����ݳ�����û�а��̣���֤��û�а����ݳ����� CaCl2 + H2O + CO2 + 2NH3��CaCO3 + 2NH4Cl b Ca(OH)2��Ba(OH)2��AgNO3��HNO3 ����
CaCl2+ 2NH3��+ 2H2O NH3 ��ʪ��ĺ�ɫʯ����ֽ����D���ڴ�������ֽ��������֤���а����ݳ�������ֽ����������֤��û�а����ݳ���������պ��Ũ����IJ���������D���ڴ������а��̣���֤���а����ݳ�����û�а��̣���֤��û�а����ݳ����� CaCl2 + H2O + CO2 + 2NH3��CaCO3 + 2NH4Cl b Ca(OH)2��Ba(OH)2��AgNO3��HNO3 ����  ��
�� ��53.5W2/143.5W1��100%
��53.5W2/143.5W1��100%
��������
I����ŨCaCl2��Һ��ͨ��NH3��CO2�������Ƶ�����̼��ƣ���ͼ��������ҩƷ��֪��A���Ʊ�������̼��B��ʢ�б���̼��������Һ���ɳ�ȥ������̼�л��е�HCl��E���Ʊ���������C���Ʊ�����̼��ƣ���ϰ�����������ˮ����ˮ�Լ��Խ��
���Ȼ����Ʒ�к�������̼�����ƣ�Ϊ�˲ⶨ�Ȼ�淋��������������ʵ�����̿�֪���õ��dz����������ԭ���غ���㡣
��1����������֪��װ��AΪ̼��������ᷴӦ�ƶ�����̼������ҩƷ��ʯ��ʯ��6mol/L���ᣬ��Ϊ��ac��
��2��Ũ�����ӷ������ɵĶ�����̼�к����Ȼ��⣬����NaHCO3��Һ�ɳ�ȥ������̼�е��Ȼ��⣻
��3��ʵ������ȡ���������Ȼ�狀��������Ƽ��ȣ������Ȼ��ơ�������ˮ������ʽΪ��2NH4Cl + Ca(OH)2![]() CaCl2+ 2NH3��+ 2H2O��
CaCl2+ 2NH3��+ 2H2O��
��4��������������ˮ����ͨ�백�����ڶ�����̼�����գ�����Ӧ��ͨ��NH3��
��5�������Ǽ������壬��ʹ��ɫʯ����ֽ�����������ܺ�Ũ���ᷴӦ���ɰ��̣���˼���D���ڴ��Ƿ��а����ݳ��ķ���Ϊ����ʪ��ĺ�ɫʯ����ֽ����D���ڴ�������ֽ��������֤���а����ݳ�������ֽ����������֤��û�а����ݳ���������պ��Ũ����IJ���������D���ڴ������а��̣���֤���а����ݳ�����û�а��̣���֤��û�а����ݳ�������
��6������ԭ���غ��֪�����Ȼ�����ɣ���������̼��ƵĻ�ѧ����ʽΪ��CaCl2+CO2+2NH3+H2O��CaCO3+2NH4Cl��
��7��������������ˮҪ��ֹ��������Ϊ��b��
��1��������Ŀ��Ϣ��֪��̼���������������������������Ʒ�Ӧ����̼�ᱵ��̼��Ƴ��������ݳ������������̼�����Ƶ��������������Ȼ�淋������Լ������������Ȼ�����������ữ����������Ӧ�����Ȼ������������ݳ�������������Ȼ�淋��������������Ȼ�淋���������������AΪCa(OH)2��Ba(OH)2��AgNO3��HNO3��
��2������BΪ���������Һ����������ù��˵ķ�����
��3�����Լ�ΪCa��OH��2��Һ������̼�غ�ù�ϵʽ��NaHCO3��CaCO3�������NaHCO3������Ϊ84W2/100g�����Ȼ�淋�����Ϊ��W1��84W2/100��g���Ȼ�淋���������Ϊ �����Լ�ΪBa(OH)2��Һ��ͬ���ɼ����Ȼ�淋���������Ϊ
�����Լ�ΪBa(OH)2��Һ��ͬ���ɼ����Ȼ�淋���������Ϊ �����Լ�ΪAgNO3��HNO3���ɹ�ϵʽNH4Cl��AgCl������Ȼ�淋���������Ϊ53.5W2/143.5W1��100%��
�����Լ�ΪAgNO3��HNO3���ɹ�ϵʽNH4Cl��AgCl������Ȼ�淋���������Ϊ53.5W2/143.5W1��100%��

����Ŀ��CO��CO2��Ӧ�ú������ǵ��������ȵ����⡣CO��ҵ�Ͽ����ڸ�¯�������������·�Ӧ��1/3Fe2O3(s) + CO(g)![]() 2/3Fe(s) + CO2(g)����֪�÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�����±���
2/3Fe(s) + CO2(g)����֪�÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�����±���
�¶�/�� | 1000 | 1150 | 1300 |
ƽ�ⳣ�� | 4.0 | 3.7 | 3.5 |
(1)�÷�Ӧ������ӦΪ_____��Ӧ(��������������������)�������CO��ƽ��ת���ʣ��ٽ� Fe2O3��ת�����ɲ�ȡ�Ĵ�ʩ��____(ѡ�����)
a���ø���Ч�Ĵ��� b�������ʯ������Ӵ����
c����ʱ���ջ��Ƴ�CO2 d������Ӧ��ϵ��ѹǿ
(2)һ�������£����ݻ�һ���������У�����CO2������Ӧ��Fe(s)��CO2(g)![]() FeO(s)��CO(g)��H>0���÷�Ӧ��ƽ�ⳣ������ʽK��_____________�����д�ʩ����ʹƽ��ʱ
FeO(s)��CO(g)��H>0���÷�Ӧ��ƽ�ⳣ������ʽK��_____________�����д�ʩ����ʹƽ��ʱ![]() �������______(ѡ����)��
�������______(ѡ����)��
a���ټ���һЩ���� b������һ����CO
c������ѹǿ d�������¶�



