��Ŀ����
Ϊ�˽�����Na2SO4��NaHCO3��NaCl�ᴿ�����Ƶô�����NaCl��Һ��ijѧ���������ͼ��ʾ��ʵ�鷽����
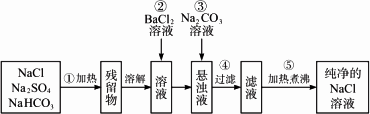
(1)������ʢ��ҩƷ��ѡ��________��(����������)
(2)������Ϊʲô����Ba(NO3)2��Һ����������________��
(3)���в����ں�����ж�![]() �ѳ�����������________��
�ѳ�����������________��
(4)�����۵�Ŀ����________��Ϊ�β��ȹ��˺��Na2CO3��Һ����������________��
(5)����Ʒ����Ƿ����ܣ���˵������________��
�𰸣�
������
������
|
�����𰸣�(1)������(2)������ ����˼·������������Ŀ��Ϣ��֪���ᴿNaCl�������ȥ���к��е����������ƺ�̼�����ƣ�����ת����ϵ֪����Ҫ��ʹ̼�����Ʒֽ⣬�������dz�ȥ̼������Ӻ���������ӣ�����Ҫ�ᴿNaCl�����Լ������ᱵ���������µ�����������������ӣ���ѡ���Ȼ����� |

��ϰ��ϵ�д�
 ȫ�ų��100��ϵ�д�
ȫ�ų��100��ϵ�д� Ӣ�ŵ��ϵ�д�
Ӣ�ŵ��ϵ�д�
�����Ŀ




