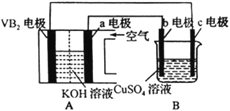
��Ŀ����
����Ŀ������������Ԫ��X��Y��Z��W��M��N ��ԭ����������������ԭ������������֮��Ϊ23��X ��ԭ�Ӱ뾶��Y ��С��X ��W ͬ���壬Z �������������ǵ��Ӳ�����3����M �ǵؿ��к�����ߵĽ���Ԫ�ء�
��1��M�����ӽṹʾ��ͼ____________��X��Y�γɵĺ�18���ӵĻ�����ĵ���ʽ____________��
�õ���ʽ��ʾ��W��Z�γɵ�ֻ�����Ӽ��Ļ�������γɹ���______________________��
��2��Y��Z��Mԭ�Ӱ뾶�ɴ�С��˳��____________����Ԫ�ط��ű�ʾ����Z���⻯���ȶ���_____N���⻯���ȶ��ԣ��������� �������� ��=����
��3��X��Z��ɵĻ������У��Ⱥ��м��Թ��ۼ��ֺ��зǼ��Թ��ۼ�����_________���ѧʽ�����˻�����ɽ����Թ�ҵ��ˮ�е�CN-����Ϊ̼���κͰ�������Ӧ�����ӷ���ʽΪ��___________________________________________________________��
���𰸡�
![]()
![]() Al��N��O �� H2O2 H2O2+CN-+OH-=CO32-+NH3��
Al��N��O �� H2O2 H2O2+CN-+OH-=CO32-+NH3��
��������
����������Ԫ��X��Y��Z��W��M��N ��ԭ��������������Z�������������ǵ��Ӳ�����3����˵��Z��OԪ�أ�M�ǵؿ��к�����ߵĽ���Ԫ�أ���M��AlԪ�أ�X��ԭ�Ӱ뾶��Y��С��X��Wͬ���壬����ԭ��������������Ĺ�ϵ�������ж�XΪHԪ����WΪNaԪ�أ�H��O��Na��Al����Ԫ������������֮��Ϊ11����Y��NԪ������������֮��Ϊ23��11=12��Y������������Ϊ5��YΪNԪ�أ�N������������Ϊ7��N��ClԪ�ء�
(1). M��AlԪ�أ�Alԭ��ʧȥ3�����ӱ��Al3+��Al3+�����ӽṹʾ��ͼΪ�� ��H��NԪ���γɵĺ�18���ӵĻ�����ΪN2H4��N2H4�ǹ��ۻ���������ʽΪ��
��H��NԪ���γɵĺ�18���ӵĻ�����ΪN2H4��N2H4�ǹ��ۻ���������ʽΪ��![]() ��Na��OԪ���γɵ�ֻ�����Ӽ��Ļ�������Na2O��Oԭ�ӵõ�2��Naԭ��ʧȥ��2�������γ�Na2O���õ���ʽ��ʾNa2O���γɹ���Ϊ��
��Na��OԪ���γɵ�ֻ�����Ӽ��Ļ�������Na2O��Oԭ�ӵõ�2��Naԭ��ʧȥ��2�������γ�Na2O���õ���ʽ��ʾNa2O���γɹ���Ϊ��![]() ��
��
�ʴ�Ϊ�� ��
��![]() ��
��![]() ��
��
(2). ͬһ������ԭ�����������ӣ�Ԫ�ص�ԭ�Ӱ뾶��С��ͬһ������ԭ�����������ӣ�Ԫ�ص�ԭ�Ӱ뾶����������N��O��Al��ԭ�Ӱ뾶�ɴ�С��˳��Ϊ��Al��N��O���ǽ�����O��Cl�����⻯����ȶ�����H2O��HCl���ʴ�Ϊ��Al��N��O������
(3). H��OԪ���γɵĻ������У��Ⱥ��м��Թ��ۼ��ֺ��зǼ��Թ��ۼ�����H2O2��H2O2�ɽ����Թ�ҵ��ˮ�е�CN������Ϊ̼���κͰ�������Ԫ�ػ��ϼ۵ı仯�����֪��H2O2�������������ݵ�ʧ�����غ��ԭ���غ㣬�÷�Ӧ�����ӷ���ʽΪ��H2O2+CN��+OH��=CO32��+NH3�����ʴ�Ϊ��H2O2��H2O2+CN��+OH��=CO32��+NH3����