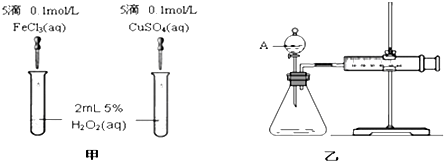
��Ŀ����
5����������ҪԪ��X��Y��Z��W��ԭ����������������Xԭ�Ӻ��������������Ǵ�����2����Y���Ȼ���YF3�������и�ԭ�Ӿ��ﵽ8�����ȶ��ṹ��Z��ͬ������ԭ�Ӱ뾶����Ԫ�أ�W���������Ϊ+7�ۣ�����˵����ȷ���ǣ�������| A�� | �����Ӱ뾶�Ĵ�С˳��Ϊ��rw��rz��ry | |
| B�� | Ԫ��W���������Ӧˮ��������Ա�Y��ǿ | |
| C�� | X��Y�γɵľ���XaYa���۵��Ӳ�ȴ�Ϊ��������ʯ�IJ��� | |
| D�� | X��W�γɵĻ������Z��W�γɵĻ�����Ļ�ѧ������ͬ |
���� ����������Ԫ��X��Y��Z��W��ԭ��������������Xԭ�Ӻ��������������Ǵ�����2������Xԭ����2�����Ӳ㣬����������Ϊ4����XΪ̼Ԫ�أ�Y�ķ�����YF3�����и�ԭ�Ӿ��ﵽ8�����ȶ��ṹ��Y����+3�ۣ�����������Ϊ��8-3=5����Y���ڢ�A�壻Z��ͬ������ԭ�Ӱ뾶����Ԫ�أ���Z��ԭ����������C����ZΪNaԪ�أ�W���������Ϊ+7�ۣ�W��ԭ����������Na����WΪClԪ�أ�Y��ԭ������С��Na����YΪNԪ�أ��ݴ˽��н��
��� �⣺����������Ԫ��X��Y��Z��W��ԭ��������������Xԭ�Ӻ��������������Ǵ�����2������Xԭ����2�����Ӳ㣬����������Ϊ4����XΪ̼Ԫ�أ�Y�ķ���������и�ԭ�Ӿ��ﵽ8�����ȶ��ṹ��Y����+3�ۣ�����������Ϊ��8-3=5����Y���ڢ�A�壻Z��ͬ������ԭ�Ӱ뾶����Ԫ�أ���Z��ԭ����������C����ZΪNaԪ�أ�W���������Ϊ+7�ۣ�W��ԭ����������Na����WΪClԪ�أ�Y��ԭ������С��Na����YΪNԪ�أ�
A�����Ӳ�Խ�࣬���Ӱ뾶Խ�࣬���Ӳ���ͬʱ���˵����Խ�����Ӱ뾶ԽС�������Ӱ뾶����Ϊ�����ӡ���С��Ϊ�����ӣ����Լ����Ӱ뾶��С˳��Ϊ��rW��rY��rZ����A����
B��WΪCl��YΪN��W������������Ӧ��ˮ��������Դ���Y��û��ָ����ۣ������ж������Դ�С��������������С�����ᣬ��B����
C��XΪC��YΪN�������γɵ�ԭ�Ӿ���C3N4�е�̼�����ۼ�����С��̼̼���������۵�Ƚ��ʯ�ߣ�Ϊ��������ʯ�IJ��ϣ���C��ȷ��
D��X��W�γɵĻ�����ΪCCl4�����й��ۼ���Z��W�γɵĻ�����ΪNaCl���������Ӽ������ߺ��л�ѧ����ͬ����D����
��ѡC��
���� ���⿼��λ�á��ṹ�����ʹ�ϵ���ۺ�Ӧ�ã���Ŀ�Ѷ��еȣ���ȷ�ƶ�Ԫ���ǽ���Ĺؼ���ע������ԭ�ӽṹ��Ԫ�������ɡ�Ԫ�����ڱ��Ĺ�ϵ����ȷ���������������뺬�л�ѧ���Ĺ�ϵ��

 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д� â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�| A�� | 11.2 LO2��O3��ɵĻ�����庬��ԭ����ΪNA | |
| B�� | ���³�ѹ�£�1.7 g NH3���еĵ�����ĿΪ NA | |
| C�� | 0.1mol/LNa2SO4��Һ�к���Na+�ĸ���Ϊ0.2NA | |
| D�� | ��״���£�22.4 L NO2��������H2O��ַ�Ӧ��ת�Ƶ�����Ϊ NA |
���밴˳�����г�1mol/L ��NH4��2SO4��Һ�и�������Ũ�ȴ�С��ϵc��NH4+����c��SO42-����c��H+����c��OH-����
��25��ʱ�����ȡ0.2mol/LHA��Һ��0.2mol/LNaOH��Һ�������ϣ����Ի�Ϻ���Һ����ı仯������û����Һ��pH=10�������Һ����ˮ�������c��H+����0.1mol/LNaOH��Һ����ˮ�������c��H+�����������������������=����
��2����������������Ӧ����1molˮ��������241.8kJ��д���÷�Ӧ���Ȼ�ѧ����ʽH2��g��+$\frac{1}{2}$O2��g���TH2O��g������H=-241.8 kJ•mol-1
��3����һ��������ܱ������У��������»�ѧ��Ӧ��CO2��g��+H2��g��?CO��g��+H2O��g�����仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���±���
| t�� | 700 | 800 | 830 | 1000 | 1200 |
| K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
�ٸ÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK=$\frac{c��CO��•c��{H}_{2}O��}{c��C{O}_{2}��•c��{H}_{2}��}$��
�ڸ÷�ӦΪ���ȷ�Ӧ��ѡ�����ȡ����ȣ���
�����жϸ÷�Ӧ�Ƿ�ﵽ��ѧƽ��״̬��������BC��
��A��������ѹǿ����
��B�����������[CO]����
��C��v����H2��=v����H2O��
��D��C��CO2��=C��CO��
| A�� | ��һ�δ�ĥ����þ������������ˮ�� | |
| B�� | ��Cl2ͨ��FeCl2��Һ�� | |
| C�� | ���̶���ļ�Ͷ�뵽ˮ�� | |
| D�� | ����ˮ�μӵ�KI������Һ�� |
| A�� | KCl | B�� | Na2S | C�� | Na2O | D�� | K2S |
| A�� | ��ˮ��������ƽ��Cl2+H2O?HCl+HClO��������AgNO3��Һ����Һ��ɫ��dz | |
| B�� | ��2HI��g��?H2��g��+I2��g����ƽ����ϵ����ѹǿ��ʹ��ɫ���� | |
| C�� | ��ӦCO+NO2?CO2+NO��H��0�������¶ȿ�ʹƽ�����淴Ӧ�����ƶ� | |
| D�� | �ϳ�NH3��Ӧ��Ϊ���NH3�IJ��ʣ�������Ӧ��ȡ�����¶ȵĴ�ʩ |

 ���÷�Ӧ������Ϊ������Ӧ��
���÷�Ӧ������Ϊ������Ӧ��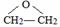 ��
��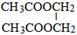 ��
��