��Ŀ����
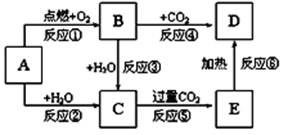
��12�֣�NaCl��һ�ֻ���ԭ�ϣ������Ʊ�һϵ�����ʣ���Ӧ���������ַ�Ӧ��������ȥ�������ǵ�ת����ϵ��ͼ��ʾ����ش��������⣺
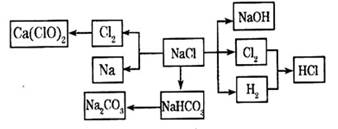
��1����ҵ�ϳ��õ������NaCl�ķ�����ȡ�����ƣ�NaCl�ۻ�ʱ�ƻ����Ӽ��Ĺ�������___________��������仯����ѧ�仯������
��2��д����ҵ����ȡHCl�Ļ�ѧ����ʽ________________________________________��
��3��д����ҵ����ȡ�ռ�����ӷ���ʽ________________________________________��
��4������ƴ���Ĺ�������Ϊ��
��NaHCO3��ȡNa2CO3�Ļ�ѧ����ʽ________________________________________��
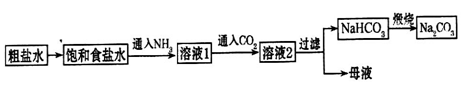
��������������ʳ��ˮ��ͨ��NH3����ͨ��CO2����Ŀ���ǣ�____________________
___________________________________________________________________________��
��5���������һʵ��֤��Na2CO3��Һ���Ա�NaHCO3��Һ�ļ���ǿ��_________________
____________________________________________________________________________

��1����ҵ�ϳ��õ������NaCl�ķ�����ȡ�����ƣ�NaCl�ۻ�ʱ�ƻ����Ӽ��Ĺ�������___________��������仯����ѧ�仯������
��2��д����ҵ����ȡHCl�Ļ�ѧ����ʽ________________________________________��
��3��д����ҵ����ȡ�ռ�����ӷ���ʽ________________________________________��
��4������ƴ���Ĺ�������Ϊ��

��NaHCO3��ȡNa2CO3�Ļ�ѧ����ʽ________________________________________��
��������������ʳ��ˮ��ͨ��NH3����ͨ��CO2����Ŀ���ǣ�____________________
___________________________________________________________________________��
��5���������һʵ��֤��Na2CO3��Һ���Ա�NaHCO3��Һ�ļ���ǿ��_________________
____________________________________________________________________________
��12�֣�ÿ��2�֣�
��1�������仯
(2)H2��Cl2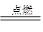 2HCl
2HCl
(3)2Cl����2H2O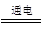 2OH����H2����Cl2��
2OH����H2����Cl2��
(4)2NaHCO3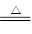 Na2CO3��H2O��CO2��
Na2CO3��H2O��CO2��
��ΪNH3��������ˮ����Һ�������ԣ���ͨ��CO2ʹ֮�����ת��ΪHCO3-
��5������ 0.1mol��L��1��������ʵ���Ũ�ȣ���������Һ������pH��Na2CO3��ҺpH����NaHCO3��Һ��˵��Na2CO3��Һ���Ա�NaHCO3��Һ�ļ���ǿ�������������𰸣�
��1�������仯
(2)H2��Cl2
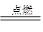 2HCl
2HCl(3)2Cl����2H2O
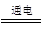 2OH����H2����Cl2��
2OH����H2����Cl2��(4)2NaHCO3
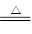 Na2CO3��H2O��CO2��
Na2CO3��H2O��CO2����ΪNH3��������ˮ����Һ�������ԣ���ͨ��CO2ʹ֮�����ת��ΪHCO3-
��5������ 0.1mol��L��1��������ʵ���Ũ�ȣ���������Һ������pH��Na2CO3��ҺpH����NaHCO3��Һ��˵��Na2CO3��Һ���Ա�NaHCO3��Һ�ļ���ǿ�������������𰸣�
��1��NaCl�ۻ�ʱ���Ӽ����ƻ�����û�������¼��������ڻ�ѧ���̣�Ϊ�����仯
��2����ҵ��һ�����������������ȼ����ȡHCl��H2��Cl2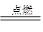 2HCl
2HCl
��3����ҵ��һ����õ��ʳ��ˮ�ķ�������ȡ�ռ2Cl����2H2O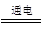 2OH����H2����Cl2��
2OH����H2����Cl2��
��4��2NaHCO3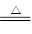 Na2CO3��H2O��CO2��
Na2CO3��H2O��CO2��
��ΪNH3��������ˮ����Һ�������ԣ���ͨ��CO2ʹ֮�����ת��ΪHCO3-
��5������ͬŨ�ȵ�����²�����Һ��pH����
��2����ҵ��һ�����������������ȼ����ȡHCl��H2��Cl2
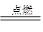 2HCl
2HCl��3����ҵ��һ����õ��ʳ��ˮ�ķ�������ȡ�ռ2Cl����2H2O
 2OH����H2����Cl2��
2OH����H2����Cl2����4��2NaHCO3
 Na2CO3��H2O��CO2��
Na2CO3��H2O��CO2����ΪNH3��������ˮ����Һ�������ԣ���ͨ��CO2ʹ֮�����ת��ΪHCO3-
��5������ͬŨ�ȵ�����²�����Һ��pH����

��ϰ��ϵ�д�
�����Ŀ
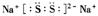 ��S32-�ĵ���ʽΪ ����֪Na2S3+2HCl = 2NaCl+H2S��+2S������д��Na2S5����ᷴӦ�����ӷ���ʽ�� ����ҵ�ϳ��õ������NaCl��Na����ʵ�ϵ���������ڵ��ƵĻ�����Ҳ���Ʊ�Na����NaOH��Na2CO3����д���������NaOH�ķ�Ӧ����ʽ�� �����������Na2CO3ʱ��CO2����������������缫��ӦʽΪ ��
��S32-�ĵ���ʽΪ ����֪Na2S3+2HCl = 2NaCl+H2S��+2S������д��Na2S5����ᷴӦ�����ӷ���ʽ�� ����ҵ�ϳ��õ������NaCl��Na����ʵ�ϵ���������ڵ��ƵĻ�����Ҳ���Ʊ�Na����NaOH��Na2CO3����д���������NaOH�ķ�Ӧ����ʽ�� �����������Na2CO3ʱ��CO2����������������缫��ӦʽΪ ��