��Ŀ����
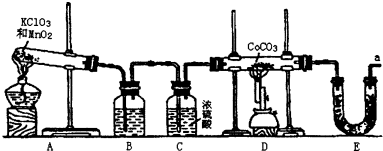
�ܣ�Co������������һ����Ҫ�Ļ���ԭ�ϣ���ҵ������CoCO3+O2��CoxOy+CO2��Ӧ��������Ӧ���ܵ������ʵ�����п���������װ������ȡ�ܵ������ﲢ�ⶨ�������ɣ�
����д���пհף�
��1��д��Aװ�õĴ��Թ������Ӧ�Ļ�ѧ����ʽ______��
��2��Eװ�õ�U�ι���ʢ�ŵ�������______��
A��P2O5B����ˮCaCl2C����ʯ��D����ˮCuSO4
��3��O3�������Ա�O2ǿ����֪�Ƶõ�O2�к���������Cl2��O3����Bװ������ʢ�ŵ�������______
A��NaOH��ҺB������NaHCO3��Һ C������NaCl��Һ D��KI��Һ
��4��ʵ�����ʱ�����ȳ�ȥAװ���еľƾ��ƣ�������______��
��5����CoCO3��ȫת��ΪCoxOy�����Ƶ�E������4.40g��D���ڲ������ʵ�������8.30g��������CoxOy�Ļ�ѧʽΪ______��
��6����ʵ��װ�ô���һ���Ƚϴ��ȱ�ݣ��������______��

����д���пհף�
��1��д��Aװ�õĴ��Թ������Ӧ�Ļ�ѧ����ʽ______��
��2��Eװ�õ�U�ι���ʢ�ŵ�������______��
A��P2O5B����ˮCaCl2C����ʯ��D����ˮCuSO4
��3��O3�������Ա�O2ǿ����֪�Ƶõ�O2�к���������Cl2��O3����Bװ������ʢ�ŵ�������______
A��NaOH��ҺB������NaHCO3��Һ C������NaCl��Һ D��KI��Һ
��4��ʵ�����ʱ�����ȳ�ȥAװ���еľƾ��ƣ�������______��
��5����CoCO3��ȫת��ΪCoxOy�����Ƶ�E������4.40g��D���ڲ������ʵ�������8.30g��������CoxOy�Ļ�ѧʽΪ______��
��6����ʵ��װ�ô���һ���Ƚϴ��ȱ�ݣ��������______��
��1���ڶ������������������������£�����طֽ������Ȼ��ء���������Ӧ����ʽΪ2KClO3
2KCl+3O2����
�ʴ�Ϊ��2KClO3
2KCl+3O2����
��2��Eװ�õ�U�ι���ʢ�ŵ�������������װ��D���ɵĶ�����̼��A��P2O5��B����ˮCaCl2��D����ˮCuSO4�������ն�����̼��C����ʯ�ҿ������ն�����̼����װ��E���Լ�Ϊ��ʯ�ң�
��ѡ��C��
��3���Ƶõ�O2�к���������Cl2��O3��Bװ������ʢ�ŵ�������������Cl2��
A��NaOH��Һ�������������������ͳ������䣬���������ܳ�������A���ϣ�
B������NaHCO3��Һ�����������������ɶ�����̼��Ӱ�������̼�����IJⶨ����B�����ϣ�
C������NaCl��Һ����������������C����
D��KI��Һ�������������������ܳ�������D���ϣ�
��ѡAD��
��4��ʵ�����ʱ�����ȳ�ȥAװ���еľƾ��ƣ��ᵼ��װ��A�е�ѹǿ���ͣ�������������
�ʴ�Ϊ��������
��5��E������4.40g�Ƕ�����̼�����������ʵ���Ϊ
=0.1mol�����ݻ�ѧʽCoCO3��֪��n��Co��=n��C��=0.1mol����Co������Ϊ0.1mol��59g/mol=5.9g��D���ڲ������ʵ�����8.30g��CoxOy��������CoxOy����Ԫ������Ϊ8.3g-5.9g=2.4g����ԭ�ӵ����ʵ���n��O��=
=0.15mol������n��Co����n��O��=0.1mol��0.15mol=2��3���ʸ��ܵ�������Ļ�ѧʽΪCo2O3��
�ʴ�Ϊ��Co2O3��
��6��װ��E�м�ʯ�ҿ������տ�����ˮ������������̼��Ӱ�������̼�����IJⶨ����Ӧ��a����һװ�м�ʯ�ҵĸ���ܣ����տ�����ˮ������������̼����ֹ����װ��E�У�
�ʴ�Ϊ��Ӧ��a����һװ�м�ʯ�ҵĸ���ܣ�
| ||
| �� |
�ʴ�Ϊ��2KClO3
| ||
| �� |
��2��Eװ�õ�U�ι���ʢ�ŵ�������������װ��D���ɵĶ�����̼��A��P2O5��B����ˮCaCl2��D����ˮCuSO4�������ն�����̼��C����ʯ�ҿ������ն�����̼����װ��E���Լ�Ϊ��ʯ�ң�
��ѡ��C��
��3���Ƶõ�O2�к���������Cl2��O3��Bװ������ʢ�ŵ�������������Cl2��
A��NaOH��Һ�������������������ͳ������䣬���������ܳ�������A���ϣ�
B������NaHCO3��Һ�����������������ɶ�����̼��Ӱ�������̼�����IJⶨ����B�����ϣ�
C������NaCl��Һ����������������C����
D��KI��Һ�������������������ܳ�������D���ϣ�
��ѡAD��
��4��ʵ�����ʱ�����ȳ�ȥAװ���еľƾ��ƣ��ᵼ��װ��A�е�ѹǿ���ͣ�������������
�ʴ�Ϊ��������
��5��E������4.40g�Ƕ�����̼�����������ʵ���Ϊ
| 4.4g |
| 44g/mol |
| 2.4g |
| 16g/mol |
�ʴ�Ϊ��Co2O3��
��6��װ��E�м�ʯ�ҿ������տ�����ˮ������������̼��Ӱ�������̼�����IJⶨ����Ӧ��a����һװ�м�ʯ�ҵĸ���ܣ����տ�����ˮ������������̼����ֹ����װ��E�У�
�ʴ�Ϊ��Ӧ��a����һװ�м�ʯ�ҵĸ���ܣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ