��Ŀ����
��16�֣��Ҷ��ᣨHOOC-COOH���������ᣬ������ˮ�����ڶ�Ԫ��ǿ�ᣬ�������Ϳ�ѧʵ�������Ź㷺����;�����ᾧ�����ɿ���H2C2O4��xH2O��ʾ��Ϊ�˲ⶨxֵ����������ʵ�飺��ȡWg���ᾧ�壬���100.00mLˮ��Һ����ȡ25.00mL�����ƵIJ�����Һ������ƿ�ڣ���������ϡH2SO4����Ũ��Ϊamol��L-1��KMnO4��Һ�ζ���
��1�����������ķ�Ӧ����ʽ��ɲ���ƽ���������Ļ�ѧ����ʽд�ڴ���ϡ� KMnO4 + H2C2O4 + K2SO4 + CO2��+ MnSO4 +
KMnO4 + H2C2O4 + K2SO4 + CO2��+ MnSO4 +
��2������ʵ������в���Ҫ�������� ������ţ���
a��������ƽ�������룬���ӣ�b����ʽ�ζ��� c����ƿ d��100mL����ƿ
e���ձ� f��©�� g����ƿ h�������� i��ҩ��
��3��ʵ���У���KMnO4��ҺӦʢװ��____ʽ�ζ����С��ζ��յ�ʱ��Һ����ɫ
�仯Ϊ ��
��4���ڵζ�����������ȥamol��L-1��KMnO4��ҺVmL���������ƵIJ�����Һ�����ʵ���Ũ��Ϊ________mol��L-1���ɴ˿ɼ���x��ֵ��___________�����ú�W��a��V�Ĵ���ʽ��ʾ��
��5����С��ͬѧ��0.02mol���ᾧ�壨H2C2O4��2H2O�����뵽100mL0.2mol/L��NaOH��Һ�г�ַ�Ӧ����÷�Ӧ�� ��Һ�����ԣ������Һ�и�����Ũ���ɴ�С��˳��Ϊ ��
��Һ�����ԣ������Һ�и�����Ũ���ɴ�С��˳��Ϊ ��
��1��2KMnO4 +5 H2C2O4 + 3H2SO4 ="==" K2SO4 + 10CO2��+2 MnSO4 + 8H2O
��2��c , f
��3���� ��Һ����ɫ��Ϊdz��ɫ�Ұ���Ӳ���ɫ
��4�� 0.1aV 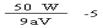 ��5�� C(Na+)��c(HC2O4-)��c(H+)��c(C2O42-) �� c(OH-)
��5�� C(Na+)��c(HC2O4-)��c(H+)��c(C2O42-) �� c(OH-)
����

 ��У����ϵ�д�
��У����ϵ�д�
 ��2009?���죩����ѧϰС����ͼװ��̽���Ҷ��ᣨHOOC-COOH�����ȷֽ�IJ��ֲ��
��2009?���죩����ѧϰС����ͼװ��̽���Ҷ��ᣨHOOC-COOH�����ȷֽ�IJ��ֲ��