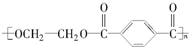��Ŀ����
PET�������ϲ������ĺϳ���ά����ṹ��ʽΪ��

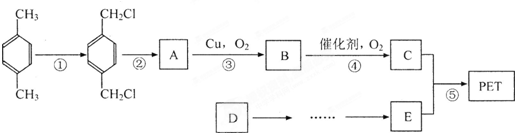
����ú�ĸ����ƷA��FΪԭ���Ʊ�PET�������Ĺ���������ͼ��ʾ������AΪ������̼Ԫ�ص���������Ϊ 90.6%���������ܶ��ǿ����ܶȵ�3.66��������ʹ���Ը��������Һ��ɫ��������ʹ��ˮ��ɫ��M����������ԭ�ӹ�ƽ�档
��ش��������⣺
(1)A�ķ���ʽΪ��____________�����з�Ӧ����Ϊ��M�� N__________ ����Ӧ��__________��
(2)��Ӧ�ٵķ�Ӧ����Ϊ��_____________��A������Ϊ__________
(3)д���л���A����һ�ȴ���Ľṹ��ʽ��________________
(4)д�����з�Ӧ�Ļ�ѧ����ʽ��
��Ӧ�ۣ�______________________��D��������������ͭ����Һ��У�______________________��
(5)P��һ��ͬϵ��X�ķ���ʽΪC3H8O2���ں˴Ź�������ͼ�г��������źŷ壬����ǿ��֮��Ϊ
2��1��1����X�Ľṹ��ʽΪ__________��
(1)A�ķ���ʽΪ��____________�����з�Ӧ����Ϊ��M�� N__________ ����Ӧ��__________��
(2)��Ӧ�ٵķ�Ӧ����Ϊ��_____________��A������Ϊ__________
(3)д���л���A����һ�ȴ���Ľṹ��ʽ��________________
(4)д�����з�Ӧ�Ļ�ѧ����ʽ��
��Ӧ�ۣ�______________________��D��������������ͭ����Һ��У�______________________��
(5)P��һ��ͬϵ��X�ķ���ʽΪC3H8O2���ں˴Ź�������ͼ�г��������źŷ壬����ǿ��֮��Ϊ
2��1��1����X�Ľṹ��ʽΪ__________��
(1)C8H10���ӳɷ�Ӧ�����۷�Ӧ
(2)NaOH��ˮ��Һ�����ȣ���ǿ��ˮ��Һ���ȣ����Զ��ױ���1��4-���ױ�
(2)NaOH��ˮ��Һ�����ȣ���ǿ��ˮ��Һ���ȣ����Զ��ױ���1��4-���ױ�
(3)

(4)CaC2+2H2O��Ca(OH)2+HC CH����
CH����
 CH����
CH����
(5)


��ϰ��ϵ�д�
 ����ѧ����ϵ�д�
����ѧ����ϵ�д�
�����Ŀ



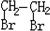

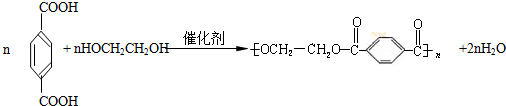

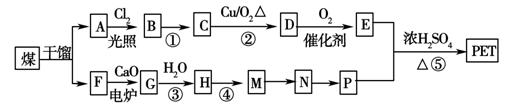
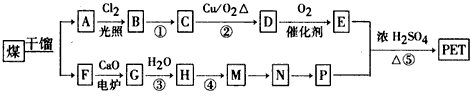
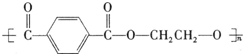 ��������ʯ�Ͳ�Ʒ�Զ��ױ������ϩ��DΪԭ�ϣ���ƺϳ�PET�Ĺ�ҵ�����������£���Ӧ�в�������Ӧ�P����δ��ʾ����
��������ʯ�Ͳ�Ʒ�Զ��ױ������ϩ��DΪԭ�ϣ���ƺϳ�PET�Ĺ�ҵ�����������£���Ӧ�в�������Ӧ�P����δ��ʾ����