��Ŀ����
����ʵ�������������ȷ����
- A.��ȡ��Һ���������У�����Һ©���л��Һ���������̨����Ȧ�Ͼ���Ƭ�̣����Ŵ��������ų��²�Һ��
- B.�ų���ʽ�ζ����м��������ʱ����������������Ƥ�ܣ�Ȼ��ѹ�����ų���Һ
- C.�Ʊ�Ħ����ʵ���У�����NH4��2SO4������Һ��FeSO4������Һ��Ϻ�������������������������������Ȼ����ȴ�����ˣ���������ˮϴȥ�����������
- D.����ˮ�Ҵ���P2O5����Al2O3����Ϻ��Ƚ�����������ͨ����ˮ�У���ˮ��Һ����ɫ
B
������A�����ݷ�Һ©���Ľṹ�ͷ�Һ���������жϣ�©�����ܷ�Һ����������
B�����ݵζ��������ݵ��ų�����������
C����NH4��2Fe��SO4��2?6H2O����Ʒ��ΪĦ���Σ�������ˮ����Ԫ��Ϊ+2�ۣ���������茶����еĽᾧˮ��������ʧȥ�����ɻ�ʹ����ֽ⣻
D������P2O5����Al2O3����ʹ��ˮ�Ҵ���ˮ������ϩ������
���A������Һ©���л��Һ���������̨����Ȧ�Ͼ���Ƭ�̣���©���Ͽڲ�������ʹ�����ۣ���С�ף�����С�ף����������ų��²�Һ�壬��A����
B������������ʹ��������б���ϣ�����ָ��ס���ܣ����ἷѹ�����飬ʹ��Һ�Ӽ����������Ӷ���Һ�������죬�ų����ݣ���B��ȷ��
C������NH4��2SO4������Һ��FeSO4������Һ��Ϻ�������������������������������Ȼ����ȴ�����˵õ��ᾧˮ�������������ˮϴ��ΪĦ����������ˮ���þƾ�����ϴȥĦ���α����ˮ��Ȼ�����ɣ���C����
D����ˮ�Ҵ���P2O5����Al2O3����Ϻ��Ƚ�������ϩ���壬ͨ����ˮ�����ӳɷ�Ӧ��ɫ����D����
��ѡB��
���������⿼����ʵ��������������ʵ��������Ʊ����������ջ��������������ô�����Ŀ��ǰ�ᣮ
������A�����ݷ�Һ©���Ľṹ�ͷ�Һ���������жϣ�©�����ܷ�Һ����������
B�����ݵζ��������ݵ��ų�����������
C����NH4��2Fe��SO4��2?6H2O����Ʒ��ΪĦ���Σ�������ˮ����Ԫ��Ϊ+2�ۣ���������茶����еĽᾧˮ��������ʧȥ�����ɻ�ʹ����ֽ⣻
D������P2O5����Al2O3����ʹ��ˮ�Ҵ���ˮ������ϩ������
���A������Һ©���л��Һ���������̨����Ȧ�Ͼ���Ƭ�̣���©���Ͽڲ�������ʹ�����ۣ���С�ף�����С�ף����������ų��²�Һ�壬��A����
B������������ʹ��������б���ϣ�����ָ��ס���ܣ����ἷѹ�����飬ʹ��Һ�Ӽ����������Ӷ���Һ�������죬�ų����ݣ���B��ȷ��
C������NH4��2SO4������Һ��FeSO4������Һ��Ϻ�������������������������������Ȼ����ȴ�����˵õ��ᾧˮ�������������ˮϴ��ΪĦ����������ˮ���þƾ�����ϴȥĦ���α����ˮ��Ȼ�����ɣ���C����
D����ˮ�Ҵ���P2O5����Al2O3����Ϻ��Ƚ�������ϩ���壬ͨ����ˮ�����ӳɷ�Ӧ��ɫ����D����
��ѡB��
���������⿼����ʵ��������������ʵ��������Ʊ����������ջ��������������ô�����Ŀ��ǰ�ᣮ

��ϰ��ϵ�д�
�����Ŀ
����ʵ������������Ӧԭ���Ľ��͡����۾���ȷ��һ���ǣ�������
| ���������� | ���ͻ���� |
| A�������ھƾ��ƻ����ϼ��ȣ��ۻ��������� | �������ۻ��������������� |
| B����ʢ��0.005mol/L FeCl3��Һ���Թ��м���5mL 0.01mol/LKSCN��Һ����Һ�ʺ�ɫ���ټ���5�α���FeCl3��Һ����ɫ���� | ����Ӧ���Ũ�ȣ���ѧƽ��������Ӧ�����ƶ� |
| C��֤��Mg��OH��2��������ת��ΪFe��OH��3 ���� |
��2mL 1mol/L NaOH��Һ���ȼ���3��1mol/L MgCl2��Һ���ټ���3��1mol/L FeCl3��Һ |
| D��ȡij��Һ���������������ữ�����ᱵ��Һ�����ְ�ɫ���� | ����Һ��һ�����д�����SO42- |
| A�������ھƾ��ƻ����ϼ��ȣ��ۻ��������� |
| B����ʢ��0.005mol/L FeCl3��Һ���Թ��м���5mL 0.01mol/LKSCN��Һ����Һ�ʺ�ɫ���ټ���5�α���FeCl3��Һ����ɫ���� |
| C��֤��Mg��OH��2��������ת��ΪFe��OH��3���� |
| D��ȡij��Һ���������������ữ�����ᱵ��Һ�����ְ�ɫ���� |
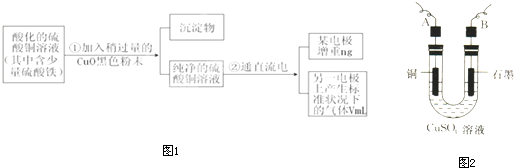

 ��4��Ŀǰ��ҵ��������̽����ŨHNO3������������Cu��ŨH2SO4��ŨHNO3��Ӧ����ȡ��Ъ���ȡ�����ŨHNO3�ķ������Ʊ�CuSO4•5H2O���¹��ա�ģ���Ʊ�װ������ͼ��ʾ��
��4��Ŀǰ��ҵ��������̽����ŨHNO3������������Cu��ŨH2SO4��ŨHNO3��Ӧ����ȡ��Ъ���ȡ�����ŨHNO3�ķ������Ʊ�CuSO4•5H2O���¹��ա�ģ���Ʊ�װ������ͼ��ʾ��