��Ŀ����
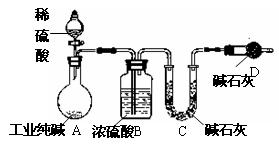
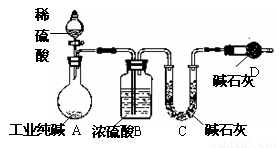
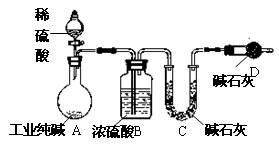
��ҵ�����г�������NaCl��Na2SO4�����ʣ�Ϊ���Բⶨij��ҵ����Ĵ��ȣ��������ͼ��ʾ��ʵ��װ�ã�����ʵ����ƣ���ش�
��1��װ��B��ʢװϡ���������������
��2��Ϊ�ﵽʵ��Ŀ�ģ�һ��ʵ��������Ӧ���г��������Ĵ���Ϊ
��3���������װ��E���ᵼ��ʵ����
��4�����һ�γ���ǰ������K����ͨ�������Ŀ����
��������1����ͼ��װ���ж�������B�з�Ӧ����̼���ƺ����ᣬ�������������ơ�ˮ��������̼��
��2�����Բⶨij��ҵ����Ĵ��ȣ���Ҫ�������������Ӧ���ɶ�����̼��������
��3��װ��E�ɷ�ֹ�����е�ˮ��������̼����D�У�
��4������K����ͨ�����壬�����ɵĶ�����̼�������D�У�
��2�����Բⶨij��ҵ����Ĵ��ȣ���Ҫ�������������Ӧ���ɶ�����̼��������
��3��װ��E�ɷ�ֹ�����е�ˮ��������̼����D�У�
��4������K����ͨ�����壬�����ɵĶ�����̼�������D�У�
����⣺��1��װ��B��ʢװϡ��������������Ƿ�Һ©����B��̼���������ᷴӦ���������ơ�ˮ��������̼����ѧ��ӦΪNa2CO3+H2SO4=Na2SO4+CO2��+H2O�����ӷ�ӦΪCO32-+2H+=CO2��+H2O���ʴ�Ϊ����Һ©����CO32-+2H+=CO2��+H2O��
��2�����Բⶨij��ҵ����Ĵ��ȣ���Ҫ�������������Ӧ���ɶ�����̼���������ⶨD��Ӧǰ������������ٲⶨ3�Σ��ʴ�Ϊ��3��
��3��װ��E�ɷ�ֹ�����е�ˮ��������̼����D�У�ʹD�ж�����̼����ƫ�����ĺ���ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
��4������K����ͨ�����壬�����ɵĶ�����̼�������D�У���Ŀ����ʹ���ƿ��������Na2CO3������CO2���ų����ʴ�Ϊ��ʹ���ƿ��������Na2CO3������CO2���ų���
��2�����Բⶨij��ҵ����Ĵ��ȣ���Ҫ�������������Ӧ���ɶ�����̼���������ⶨD��Ӧǰ������������ٲⶨ3�Σ��ʴ�Ϊ��3��
��3��װ��E�ɷ�ֹ�����е�ˮ��������̼����D�У�ʹD�ж�����̼����ƫ�����ĺ���ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
��4������K����ͨ�����壬�����ɵĶ�����̼�������D�У���Ŀ����ʹ���ƿ��������Na2CO3������CO2���ų����ʴ�Ϊ��ʹ���ƿ��������Na2CO3������CO2���ų���
���������⿼�����ʺ�����ʵ�鷽������ƣ�����װ�õ����ü�ʵ��ԭ��Ϊ���Ĺؼ���ע��ʵ��˼ά�������Լ��������Ŀ��飬��ѧ������Ҫ��ϸߣ���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
 (1��װ����ʢװϡ��������������� ��
(1��װ����ʢװϡ��������������� ��