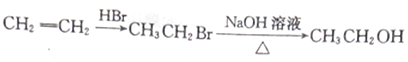��Ŀ����
����Ŀ���±���Ԫ�����ڱ���һ���֣���֪��Ϊ������Ԫ�أ��䵥��Ϊ����ɫ���壬�ݱ��ش��й����⣺
�� | �� | ||||||
�� | �� | �� | �� | �� | |||
�� | �� |
(1)����Ԫ�آ��ԭ�ӽṹʾ��ͼ __________________
(2)����ЩԪ���У�����õķǽ���Ԫ���� ______�� ����õ�Ԫ����_____��дԪ�ط��� ����
(3)����ЩԪ�ص�����������Ӧˮ�����У� ������ǿ���� __________(д��ѧʽ)�������Ե�����������____________(д��ѧʽ)��д������֮�䷴Ӧ�����ӷ���ʽ�� ______________________________
��4���ڢ�����У���ѧ���ʽϻ��õ���________��дԪ�ط��� ����д��������֤�ý��۵�һ����ѧ��Ӧ��ʽ _________________________________________��
���𰸡� 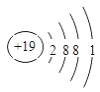 F Ar KOH Al��OH��3 Al��OH��3 +OH- =AlO2- + 2H2O Cl Cl2+2NaBr=Br2+2HCl
F Ar KOH Al��OH��3 Al��OH��3 +OH- =AlO2- + 2H2O Cl Cl2+2NaBr=Br2+2HCl
����������֪��Ϊ������Ԫ�أ��䵥��Ϊ����ɫ���壬�����S�����Ԣ���Li������F������Na������Al������Cl������Ar������K������Br��
(1)KԪ�ص�ԭ�ӽṹʾ��ͼΪ ��(2)����ЩԪ���У�����õķǽ���Ԫ����F�� ����õ�Ԫ����ϡ������Ar�� (3)����ЩԪ�ص�����������Ӧˮ�����У� ������ǿ����KOH�������Ե�����������Al(OH)3������֮�䷴Ӧ�����ӷ���ʽΪAl��OH��3 +OH- =AlO2- + 2H2O����4��ͬ������ԭ������������ǽ����Լ��������ڢ�����У���ѧ���ʽϻ��õ���Cl�������ܰ����û�����������֤�ý��ۣ���Ӧ�Ļ�ѧ��Ӧ��ʽΪ Cl2+2NaBr��Br2+2HCl��
��(2)����ЩԪ���У�����õķǽ���Ԫ����F�� ����õ�Ԫ����ϡ������Ar�� (3)����ЩԪ�ص�����������Ӧˮ�����У� ������ǿ����KOH�������Ե�����������Al(OH)3������֮�䷴Ӧ�����ӷ���ʽΪAl��OH��3 +OH- =AlO2- + 2H2O����4��ͬ������ԭ������������ǽ����Լ��������ڢ�����У���ѧ���ʽϻ��õ���Cl�������ܰ����û�����������֤�ý��ۣ���Ӧ�Ļ�ѧ��Ӧ��ʽΪ Cl2+2NaBr��Br2+2HCl��

����Ŀ��Һ��ʯ������Ϊȼ�������ձ������м�ͥ�����Ǻ����������ʵĻ�������ڳ�ѹ������Щ���ʵķе����±���ʾ��
�������� | ���� | ���� | ���� | ���� | ���� |
�е�/�� | ��88.6 | ��42.1 | ��0.5 | 36.1 | 69.2 |
�ڳ�����ʹ����������ų�ʱ����ƿ�г�ʣ��һЩҺ̬��������Щ�������п�����(����)
A. ���顢����Ͷ��� B. ����ͱ���
C. ֻ������ D. ����ͼ���