��Ŀ����
��ͼ1��ʵ������ȡ������װ��ͼ����ش�

��1��ʵ������ȡ�����Ļ�ѧ����ʽ��______��
��2����ͬѧ�����Ӽ�ȡʪ��ĺ�ɫʯ����ֽ�����Թܿڣ��ɹ۲쵽�����õ��Ľ�����______��
��3�����ﰱ����ѡ�õ��Լ���______������ţ���
�ټ�ʯ�ҡ����� ��Ũ���ᡡ����������������Һ
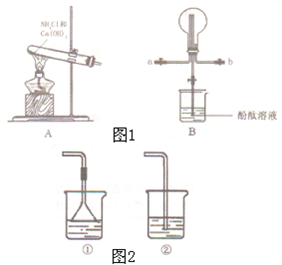
��4��ͼ2��ʵ������ȡ������һЩװ�ú�ѡ�õ��Լ������в���ȷ����______������ţ���
��5���������İ���ͨ���Ȼ�����Һ�У�һ��ʱ��۲쵽��ʵ��������______��������Ӧ�����ӷ���ʽ��______��
�⣺��1��ʵ�����Ʊ������������Ȼ�狀��������ƹ�����ȷ�Ӧ���ɣ���Ӧ�Ļ�ѧ����ʽΪ��2NH4Cl+Ca��OH��2 2NH3��+2H2O+CaCl2��
2NH3��+2H2O+CaCl2��
�ʴ�Ϊ��2NH4Cl+Ca��OH��2 2NH3��+2H2O+CaCl2��
2NH3��+2H2O+CaCl2��
��2��������ʹʪ��ĺ�ɫʯ����ֽ�����������Ӽ�ȡʪ��ĺ�ɫʯ����ֽ�����Թܿڣ��ɹ۲쵽�����õ��Ľ����ǣ�ʪ��ĺ�ɫʯ����ֽ������֤�������˰�����
�ʴ�Ϊ��ʪ��ĺ�ɫʯ����ֽ������֤�������˰�����
��3�������Ǽ������壬���������Ը��������������Һ���ټ�ʯ�ҿ��Ը��ﰱ������Ũ����Ͱ�����Ӧ���ܸ��ﰱ����������������Һ�������������
�ʴ�Ϊ���٣�
��4�����ټ���Ũ��ˮ����Һ��һˮ�ϰ��ֽ����ɰ��������������Ʊ��������ʢ���ȷ��
�ڹ��������ˮ���ɵ���ը���Թܣ��ʢڴ���
��̼��������ȷֽ����ɰ�����������̼��ˮ���Ƶõİ����������ʢ۴���
�ʴ�Ϊ���ڢۣ�
��5���������İ���ͨ���Ȼ�����Һ�У���Ӧ��������������������Ӧ�����ӷ���ʽΪ��Al3+3NH3+3H2O=Al��OH��3��+3NH4+��
�ʴ�Ϊ�����ɰ�ɫ������Al3+3NH3+3H2O=Al��OH��3��+3NH4+��
��������1��ʵ�����Ʊ������������Ȼ�狀��������ƹ�����ȷ�Ӧ���ɣ�
��2��������ʹʪ��ĺ�ɫʯ����ֽ������
��3�������Ǽ������壬���������Ը��������������Һ��
��4���ټ���Ũ��ˮһˮ�ϰ��ֽ����ɰ�����
�ڹ��������ˮ���ɵ���ը���Թܣ�
��̼��������ȷֽ����ɰ�����������̼��ˮ���Ƶõİ���������
��5�������İ���ͨ���Ȼ�����Һ�з�Ӧ������������������
���������⿼��ʵ�����Ʊ�������װ�ú��Լ�ѡ��������飬���ջ����ǹؼ�����Ŀ�ϼ�
 2NH3��+2H2O+CaCl2��
2NH3��+2H2O+CaCl2���ʴ�Ϊ��2NH4Cl+Ca��OH��2
 2NH3��+2H2O+CaCl2��
2NH3��+2H2O+CaCl2����2��������ʹʪ��ĺ�ɫʯ����ֽ�����������Ӽ�ȡʪ��ĺ�ɫʯ����ֽ�����Թܿڣ��ɹ۲쵽�����õ��Ľ����ǣ�ʪ��ĺ�ɫʯ����ֽ������֤�������˰�����
�ʴ�Ϊ��ʪ��ĺ�ɫʯ����ֽ������֤�������˰�����
��3�������Ǽ������壬���������Ը��������������Һ���ټ�ʯ�ҿ��Ը��ﰱ������Ũ����Ͱ�����Ӧ���ܸ��ﰱ����������������Һ�������������
�ʴ�Ϊ���٣�
��4�����ټ���Ũ��ˮ����Һ��һˮ�ϰ��ֽ����ɰ��������������Ʊ��������ʢ���ȷ��
�ڹ��������ˮ���ɵ���ը���Թܣ��ʢڴ���
��̼��������ȷֽ����ɰ�����������̼��ˮ���Ƶõİ����������ʢ۴���
�ʴ�Ϊ���ڢۣ�
��5���������İ���ͨ���Ȼ�����Һ�У���Ӧ��������������������Ӧ�����ӷ���ʽΪ��Al3+3NH3+3H2O=Al��OH��3��+3NH4+��
�ʴ�Ϊ�����ɰ�ɫ������Al3+3NH3+3H2O=Al��OH��3��+3NH4+��
��������1��ʵ�����Ʊ������������Ȼ�狀��������ƹ�����ȷ�Ӧ���ɣ�
��2��������ʹʪ��ĺ�ɫʯ����ֽ������
��3�������Ǽ������壬���������Ը��������������Һ��
��4���ټ���Ũ��ˮһˮ�ϰ��ֽ����ɰ�����
�ڹ��������ˮ���ɵ���ը���Թܣ�
��̼��������ȷֽ����ɰ�����������̼��ˮ���Ƶõİ���������
��5�������İ���ͨ���Ȼ�����Һ�з�Ӧ������������������
���������⿼��ʵ�����Ʊ�������װ�ú��Լ�ѡ��������飬���ջ����ǹؼ�����Ŀ�ϼ�

��ϰ��ϵ�д�
�����Ŀ

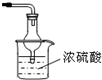
 ij��ѧʵ��С��ͬѧ������ͼ1װ���Ʊ���������̽�����������ʣ�������������ȥ����
ij��ѧʵ��С��ͬѧ������ͼ1װ���Ʊ���������̽�����������ʣ�������������ȥ���� �ң�
�ң� ��
��



