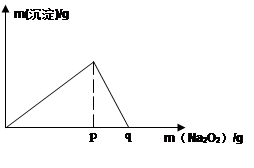��Ŀ����
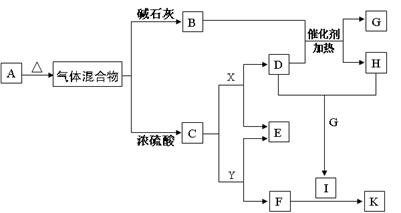
��11�֣���ͼ����A~K����a��b��c��d��e��f����Ԫ����ɡ�a��b��c��d��e��fΪԭ��������������Ķ�����Ԫ�أ���a��b��c��d����Ԫ���γ���ʽ��A��b�������������ǵ��Ӳ�����2������d��eԪ�ؿ��γ��������ӻ�������������������ӵĸ����ȶ�Ϊ2 : 1������һ��ΪX��B��C��DΪ���������壻FΪ��ɫ��״���ʡ������ֲ���δ�г���

��1������A�Ļ�ѧʽ_______________
��2��Y����Һ�� �ԣ�����ԡ� �������ԡ� �����ԡ����������ӷ�Ӧ����ʽ����ԭ��
��3������ҺK�������գ��õ��Ĺ������Ϊ ���˹���������;�� �����һ�����ɣ�
��4��B��H��һ�������·�Ӧ����c���ʺ�G����Ӧ�����������뻹ԭ�������ʵ���֮��Ϊ
��5�������£���0.1mol/L��K��Һ�м���Na2O2������Na2O2���������������������������ͼ��ʾ��ϵ
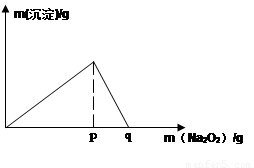
�ٵ���ҺpH= ����ʼ���ɳ���(��֪���ɳ�����Ksp=1��10-31 )
��p��ʱ����������ų���������ʵ���֮��Ϊ
�۴ӿ�ʼ��Na2O2������q������������У��ܷ�Ӧ�����ӷ���ʽΪ ��
��1�� NH4HCO3��1�֣�
��2�����ԣ�1�֣� AlO2��+2H2O Al(OH)3+OH����1�֣�
Al(OH)3+OH����1�֣�
��3��Al2O3 ��1�֣� �����ͻ���� (�����𰸺�������) ��1�֣�
��4��2:3 ��1�֣�
��5����4 ��1�֣� ��4:3 ��2�֣� ��Al3++2Na2O2=AlO2��+O2��+4Na+��2�֣�
��������
���������b�������������ǵ��Ӳ�����2������b����ΪC ��S
��S ����a��b��c��d��e��fΪԭ��������������Ķ�����Ԫ�أ���bԭ��������Сֻ��ΪC��d��eԪ�ؿ��γ��������ӻ�������������������ӵĸ����ȶ�Ϊ2
: 1����dֻ����OԪ�أ�eֻ����NaԪ�أ�X����ΪNa2O2��Na2O��a��b��c��d����Ԫ���γ���ʽ��A����AΪNH4HCO3��NH4HCO3=NH3+H2O+CO2,��CΪCO2��BΪNH3��XΪNa2O2,DΪO2,GΪH2O��HΪNO��IΪNO2��FΪ��ɫ��״����,��FΪAl(OH)3,��YΪNaAlO2��KΪAl��NO3��3��
����a��b��c��d��e��fΪԭ��������������Ķ�����Ԫ�أ���bԭ��������Сֻ��ΪC��d��eԪ�ؿ��γ��������ӻ�������������������ӵĸ����ȶ�Ϊ2
: 1����dֻ����OԪ�أ�eֻ����NaԪ�أ�X����ΪNa2O2��Na2O��a��b��c��d����Ԫ���γ���ʽ��A����AΪNH4HCO3��NH4HCO3=NH3+H2O+CO2,��CΪCO2��BΪNH3��XΪNa2O2,DΪO2,GΪH2O��HΪNO��IΪNO2��FΪ��ɫ��״����,��FΪAl(OH)3,��YΪNaAlO2��KΪAl��NO3��3��
���㣺Ԫ�����ڱ����ϳɰ�����ʽ�������������ʣ��ƵĻ�����
�������ؼ������������Ϣ�ҵ�ͻ�Ƶ㣬�����θ����Ƶ��Ŀ�ͼ����δ֪������ͻ�Ƶ�ܶ࣬����˵��ɫ��״����Ӧ������������������һЩ���Ե���Ϣ����ֱ���Ƴ�δ֪�