��Ŀ����
ˮ������֮Դ��Ҳ�ǻ�ѧ��Ӧ�е����ǣ���ش��������⣺
I��ˮ��һ�ֵ���ʣ�����������������������ͬ��������������뷽��ʽΪ______��
�������෴Ӧ��H2O���ݲ�ͬ�ġ���ɫ����������ѧ֪ʶ��д���йط�Ӧ�Ļ�ѧ����ʽ�����ӷ���ʽ��
��1��H2O�����û���Ӧ������X+W��Y+V��
��֪X��Y�ֱ��Ƕ���������Ԫ���γɵ����ֵ��ʣ�W��V�ǻ����
��W��ˮ������ԭ�����÷�Ӧ�Ļ�ѧ����Ϊ______��
��V��ˮ����ѧ����ʽΪ______��
��2��ˮ��������ԭ��Ӧ�Ȳ���������Ҳ���ǻ�ԭ����
A��B����ѧ��ѧ�����������ɶ�����Ԫ����ɵ���ɫ���壬���Ǿ�����ˮ����������ԭ��Ӧ����ˮ�Ȳ���������Ҳ���ǻ�ԭ����д��A��B��ˮ��Ӧ�Ļ�ѧ����ʽ��
��A+H2O______��
��B+H2O______��
��3��ij���ʻ�ѧʽΪNH5���������ǹ�̬������ˮ���ҷ�Ӧ�ų��������壮��NH5�еĸ�ԭ�Ӿ�����ϡ��������ȶ��ṹ�������ж�NH5��ˮ�ķ�Ӧ������ȷ����______����ѡ���
A��NH5��ˮ��Ӧʱ��NH5���������� B��NH5��ˮ��Ӧʱ��NH5�������������ǻ�ԭ��
C��NH5��ˮ��Ӧʱ��NH5�ǻ�ԭ���� D��NH5��NH3����ˮ����Һ���ʼ��ԣ�
��1����W��ˮ������ԭ������X�������������ǵ��ʣ��ܺ�ˮ��Ӧ�����������Ķ�����Ԫ�ص����Ƿ�����������ˮ��Ӧ�Ļ�ѧ��Ӧ����ʽΪ��2F2+2H2O�TO2+4HF��
�ʴ�Ϊ��2F2+2H2O�TO2+4HF��
��V��ˮ����WΪ�⻯���XΪO2�������������û���ӦΪO2+2H2S=2H2O+2S�����ʴ�Ϊ��O2+2H2S=2H2O+2S����
��2�������ɶ�����Ԫ����ɵ���ɫ������Cl2��NO2�����Ǿ�����ˮ����������ԭ��Ӧ����ˮ�Ȳ���������Ҳ���ǻ�ԭ����
��Cl2��ˮ��Ӧ����HCl��HClO����ѧ����ʽΪCl2+H2O=HCl+HClO���ʴ�Ϊ��Cl2+H2O=HCl+HClO��
��NO2��ˮ��Ӧ����NO��HNO3����ѧ����ʽΪ3NO2+H2O=2HNO3+NO���ʴ�Ϊ��3NO2+H2O=2HNO3+NO��
��3��NH5��ˮ�����ķ�Ӧ����ʽΪ��NH5+H2O=NH3?H2O+H2����NH3��H2O=NH3��+H2O����Ӧ�У�NH5�е�HԪ��ʧ���ӣ�H2O�е�HԪ�صõ��ӣ�����NH5�ǻ�ԭ����H2O����������NH5��NH3����ˮ�����ɰ�ˮ��Һ��������Һ���ʼ��ԣ���ѡCD��
������I��ˮ�����OH-��H3O+���ӣ����ߵ���������ȣ�
��1����W��ˮ������ԭ������X�������������ǵ��ʣ��ܺ�ˮ��Ӧ�����������Ķ�����Ԫ�ص����Ƿ������ݴ�д����Ӧ����ʽ��
��V��ˮ����WΪ�⻯���XΪO2��
��2�������ɶ�����Ԫ����ɵ���ɫ������Cl2��NO2�����Ǿ�����ˮ����������ԭ��Ӧ����ˮ�Ȳ���������Ҳ���ǻ�ԭ����
��3��NH5�еĸ�ԭ�Ӿ�����ϡ��������ȶ��ṹ�����ԣ�NH5����笠����Ӻ����������γɵ����ӻ�����������Ȼ�什ṹ����ˮ��Ӧ�����������壬NH5+H2O=NH3?H2O+H2����NH3��H2O=NH3��+H2O������Ԫ�ػ��ϼ��ж��������ͻ�ԭ����
���������⿼��������ԭ��Ӧ�Լ�������ƶϵ�֪ʶ����Ŀ��Ϊ�ۺϣ��Ѷ��еȣ�����ע�ⳣ��Ԫ�ػ�����֪ʶ�Ļ��ۣ��ι̰��ջ���֪ʶ��������Ŀ�ɽ������

��1��ˮ��������ԭ���ڻ�̬ʱ��������Ų�ʽΪ
��2��H2O��������ԭ�Ӳ�ȡ����
��3��ˮ�������õ�һ��H+�γ�ˮ�������ӣ�H3O+�������������̵�������������������
A����ԭ�ӵ��ӻ����ͷ����˸ı� B��������״�����˸ı�
C��ˮ�����Ա������Ļ�ѧ���� D�����еļ��Ƿ����˸ı�
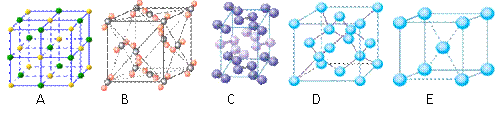
��4���������ơ��⡢���ʯ���ɱ����Ȼ��ƾ���ľ���ͼ��δ��˳����������ľ���
������ͬ����
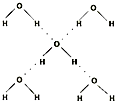
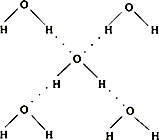
��5���ڱ������У�ÿ��ˮ���������ڵ�4��ˮ�����γ��������ͼ��ʾ������֪������������51kJ/mol��������⣬ˮ���Ӽ仹���ڷ��»�����11kJ/mol�����������������ġ����ܡ���
��6������ɫ����ˮCuSO4�ܽ���ˮ�У���Һ����ɫ������Ϊ������һ�ֳ���ɫ��������ӣ���д�����ɴ�������ӵ����ӷ���ʽ��
��7����֪����Ԫ�صĵ縺�����ݣ�H��2.1��O��3.5��F��4.0��OF2��ˮ������ṹ���ƣ���ˮ���ӵļ��Ա�OF2ǿ�ö࣬��ԭ���У���OF2����ԭ���������Թ¶Ե��ӣ�������FһO���й��õ��Ӷ�ƫ��F�������ļ��ԣ��ڴӵ縺���Ͽ���
��8�������±����ݣ���д�������߸����ԵĽ��ۣ�
| ���� | ���� ��kJ/mol�� |
���� ��pm�� |
���� | ���� | ���� | �۵㣨�棩 | �е㣨�棩 |
| H-C | 413 | 109 | 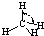 |
109.5�� | ���� | -183.7 | -128.0 |
| H-N | 391 | 101 | 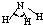 |
107�� | �� | -77.7 | -33.3 |
| H-O | 467 | 96 | 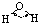 |
104.5�� | ˮ | 0.0 | 100.0 |
ˮ������֮Դ��Ҳ��һ�ֳ��õ��Լ�����ش��������⣺
��1��ˮ��������ԭ���ڻ�̬ʱ��������Ų�ʽΪ___ _______��
��2��H2O��������ԭ�Ӳ�ȡ���� �ӻ���
��3��ˮ�������õ�һ��H���γ�ˮ�������ӣ�H3O���������������̵������������������� ��
| A����ԭ�ӵ��ӻ����ͷ����˸ı� | B��������״�����˸ı� |
| C��ˮ�������ӷ��ӹ����������� | D�����еļ��Ƿ����˸ı� |
ˮ������֮Դ��Ҳ��һ�ֳ��õ��Լ�����ش��������⣺
��1��ˮ��������ԭ���ڻ�̬ʱ��������Ų�ʽΪ ��
��2��H2O��������ԭ�Ӳ�ȡ���� �ӻ���
��3��ˮ�������õ�һ��H���γ�ˮ�������ӣ�H3O���������������̵������������������� ��
A����ԭ�ӵ��ӻ����ͷ����˸ı� B�����Ŀռ乹�ͷ����˸ı�
C�����еļ��Ƿ����˸ı�
��4���������ơ��⡢���ʯ���ɱ����Ȼ��ƾ���ľ���ͼ(δ��˳������)������ľ���������ͬ����______��(������Ӧ�ı����д)
 |

��5���ڱ������У�ÿ��ˮ���������ڵ�4��ˮ�����γ����(��ͼ��ʾ)����֪������������51 kJ/mol��������⣬ˮ���Ӽ仹���ڷ��»���(11 kJ/mol)���������������ġ����ܡ���_________kJ/mol��
��6������ɫ����ˮCuSO4�ܽ���ˮ�У���Һ����ɫ������Ϊ������һ�ֳ���ɫ��������ӡ���д�����ɴ�������ӵ����ӷ���ʽ�� ��