��Ŀ����
��1����һ�ֽ����س�ʯ��K2Al2Si6O16�����仯ѧʽΪд�����������ʽΪ
��2����Ӧ3Cu+8HNO3=3Cu��NO3��2+2NO��+4H2O �����ӷ���ʽΪ��
ÿ����11.2L��״���µ����壬����ԭ��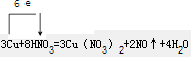
 ��
��
��3����֪A��B��C��D��E��F�Ǻ���ͬһ��Ԫ�صĻ��������F����ʹ��ɫʪ��ʯ����ֽ���������壬����֮���ܷ������·�Ӧ��A+H2O��B+C ��C+F��D ��D+NaOH
F+E+H2Oд��A��B��C�Ļ�ѧʽA
K2O?Al2O3?6SiO2
K2O?Al2O3?6SiO2
����2����Ӧ3Cu+8HNO3=3Cu��NO3��2+2NO��+4H2O �����ӷ���ʽΪ��
3Cu+8H++2NO3?=3Cu2++2NO��+4H2O
3Cu+8H++2NO3?=3Cu2++2NO��+4H2O
��ÿ����11.2L��״���µ����壬����ԭ��
����
����
�������ƣ�������Ϊ31.5g
31.5g
���õ�����ʧ���Ӹ�������1��1
1��1
���������뱻��ԭ��ԭ�ӵĸ�����Ϊ3��2
3��2
�����á������š�����÷�Ӧ����ת�Ʒ������Ŀ��

��3����֪A��B��C��D��E��F�Ǻ���ͬһ��Ԫ�صĻ��������F����ʹ��ɫʪ��ʯ����ֽ���������壬����֮���ܷ������·�Ӧ��A+H2O��B+C ��C+F��D ��D+NaOH
| �� |
NO2
NO2
BNO
NO
CHNO3
HNO3
��д����Ӧ�۵����ӷ���ʽNH4++OH-
NH3+H2O
| ||
NH4++OH-
NH3+H2O
��
| ||
��������1��������д�����������ʽ�ǰ��ջ��ý�����������ϻ��ý�����������������衢ˮ��˳������д������ѭԭ���غ㣻
��2�����ʡ�ˮ�������ڻ�ѧ��Ӧ��дΪ���ӷ�ӦʱӦ������ѧʽ�����û��ϼ����仯����������������ԭ����ת�Ƶĵ�������
��3��F����ʹ��ɫʪ��ʯ����ֽ���������壬��FΪ������A��B��C��D��E��F�ж�����NԪ�أ�����ת����������
��2�����ʡ�ˮ�������ڻ�ѧ��Ӧ��дΪ���ӷ�ӦʱӦ������ѧʽ�����û��ϼ����仯����������������ԭ����ת�Ƶĵ�������
��3��F����ʹ��ɫʪ��ʯ����ֽ���������壬��FΪ������A��B��C��D��E��F�ж�����NԪ�أ�����ת����������
����⣺��1���س�ʯ�����ΪK2Al2Si6O16������������K��Al��������д˳��ԭ���غ㣬��ѧʽΪд�����������ʽΪK2O?Al2O3?6SiO2���ʴ�Ϊ��K2O?Al2O3?6SiO2��
��2����Ӧ3Cu+8HNO3=3Cu��NO3��2+2NO��+4H2O ��д�����ӷ�ӦʱCu��NO��H2OӦ������ѧʽ�������ӷ�ӦΪ3Cu+8H++2NO3?=3Cu2++2NO��+4H2O������11.2L��״���µ����壬
NO�����ʵ���Ϊ0.5mol��������NԪ�صĻ��ϼ۽��ͣ������ᱻ��ԭ��������Ϊ0.5mol��63g/mol=31.5g��������ԭ��Ӧ�е�ʧ�����غ㣬��õ�����ʧ���Ӹ�������1��1��
Cu�����������ᱻ��ԭ�������ӷ���ʽ��֪���������뱻��ԭ��ԭ�ӵĸ�����Ϊ3��2���÷�ӦCuʧȥ���Ӹ�N����ת��6e�������ŷ�����÷�Ӧ����ת�Ʒ������ĿΪ
 ���ʴ�Ϊ��3Cu+8H++2NO3?=3Cu2++2NO��+4H2O�����31.5g��1��1��3��2��
���ʴ�Ϊ��3Cu+8H++2NO3?=3Cu2++2NO��+4H2O�����31.5g��1��1��3��2�� ��
��
��3��F����ʹ��ɫʪ��ʯ����ֽ���������壬��FΪ������A��B��C��D��E��F�ж�����NԪ�أ���A+H2O��B+CӦΪNO2+H2O��NO+HNO3����C+F��DӦΪHNO3+NH3��NH4NO3��
��D+NaOH
F+E+H2OӦΪNH4NO3+NaOH
NH3+NaNO3+H2O�������ӷ�ӦΪNH4++OH-
NH3+H2O��
�ʴ�Ϊ��NO2��NO��HNO3��NH4++OH-
NH3+H2O��
��2����Ӧ3Cu+8HNO3=3Cu��NO3��2+2NO��+4H2O ��д�����ӷ�ӦʱCu��NO��H2OӦ������ѧʽ�������ӷ�ӦΪ3Cu+8H++2NO3?=3Cu2++2NO��+4H2O������11.2L��״���µ����壬
NO�����ʵ���Ϊ0.5mol��������NԪ�صĻ��ϼ۽��ͣ������ᱻ��ԭ��������Ϊ0.5mol��63g/mol=31.5g��������ԭ��Ӧ�е�ʧ�����غ㣬��õ�����ʧ���Ӹ�������1��1��
Cu�����������ᱻ��ԭ�������ӷ���ʽ��֪���������뱻��ԭ��ԭ�ӵĸ�����Ϊ3��2���÷�ӦCuʧȥ���Ӹ�N����ת��6e�������ŷ�����÷�Ӧ����ת�Ʒ������ĿΪ
 ���ʴ�Ϊ��3Cu+8H++2NO3?=3Cu2++2NO��+4H2O�����31.5g��1��1��3��2��
���ʴ�Ϊ��3Cu+8H++2NO3?=3Cu2++2NO��+4H2O�����31.5g��1��1��3��2�� ��
����3��F����ʹ��ɫʪ��ʯ����ֽ���������壬��FΪ������A��B��C��D��E��F�ж�����NԪ�أ���A+H2O��B+CӦΪNO2+H2O��NO+HNO3����C+F��DӦΪHNO3+NH3��NH4NO3��
��D+NaOH
| �� |
| �� |
| ||
�ʴ�Ϊ��NO2��NO��HNO3��NH4++OH-
| ||
����������ΪС�ۺϣ�������֪ʶ�㣬��ȷ�����ε���ɡ�������ԭ��Ӧ��Ԫ�صĻ��ϼ۱仯�����ӷ���ʽ����д������������֮���ת�����ɽ���ѶȲ���

��ϰ��ϵ�д�
 ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
�����Ŀ
 ��3����֪A��B��C��D��E��F�Ǻ���ͬһ��Ԫ�صĻ��������F����ʹ��ɫʪ��ʯ����ֽ���������壬����֮���ܷ������·�Ӧ
��3����֪A��B��C��D��E��F�Ǻ���ͬһ��Ԫ�صĻ��������F����ʹ��ɫʪ��ʯ����ֽ���������壬����֮���ܷ������·�Ӧ  ��3����֪A��B��C��D��E��F�Ǻ���ͬһ��Ԫ�صĻ��������F����ʹ��ɫʪ��ʯ����ֽ���������壬����֮���ܷ������·�Ӧ
��3����֪A��B��C��D��E��F�Ǻ���ͬһ��Ԫ�صĻ��������F����ʹ��ɫʪ��ʯ����ֽ���������壬����֮���ܷ������·�Ӧ