��Ŀ����
��ͼ��ʾ��Ԫ�����ڱ���1��20���Ҳ�ͬ����Ԫ����ɵĵ��ʼ�������֮��ת����ϵ�������е�ˮ����ȥ��������AΪ���ʣ���ʵ�����У����ù���B����C������ȡ����F��F��G�����Ԫ����ͬ��G��I����������������ͬ����ش�
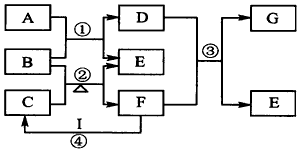
��1��д��B��G�Ļ�ѧʽB_________��G_________��
��2��д��C�ĵ���ʽ_________����Ӧ�ܵ�ʵ������Ϊ__________________��
��3�������Ư�����õ�D��Һ����I��ϡ��Һ��д����Ӧ�����ӷ���ʽ��___________________________
��4����D����Һ����I��Ũ��Һ��A���ɣ��䷴Ӧ�����ӷ���ʽΪ��___________________________��
��5��д����Ӧ�۵Ļ�ѧ����ʽ___________________________��
��2��д��C�ĵ���ʽ_________����Ӧ�ܵ�ʵ������Ϊ__________________��
��3�������Ư�����õ�D��Һ����I��ϡ��Һ��д����Ӧ�����ӷ���ʽ��___________________________
��4����D����Һ����I��Ũ��Һ��A���ɣ��䷴Ӧ�����ӷ���ʽΪ��___________________________��
��5��д����Ӧ�۵Ļ�ѧ����ʽ___________________________��
��1��Ca(OH)2��N2H4
��2�� ������
������
��3��ClO-+H+==HClO
��4��ClO-+Cl-+2H+==Cl2��+H2O
��5��4NH3+Ca(ClO)2==2N2H4+CaCl2+2H2O
��2��
 ������
��������3��ClO-+H+==HClO
��4��ClO-+Cl-+2H+==Cl2��+H2O
��5��4NH3+Ca(ClO)2==2N2H4+CaCl2+2H2O

��ϰ��ϵ�д�
 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�
�����Ŀ



