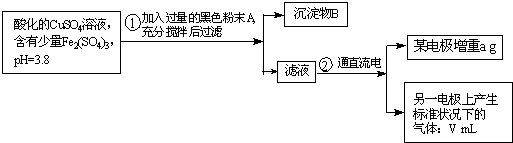
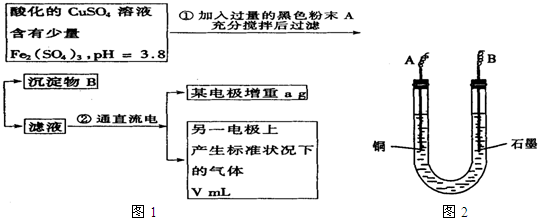
��Ŀ����
��֪��pHΪ4��5����Һ�У�Cu2+������ˮ�⣬��Fe3+������ȫˮ�⣮ijѧ�����õ��CuSO4��Һ�ķ����ⶨͭ�����ԭ����������ʵ��������ͼ1��ʾ��

�Իش��������⣺
��1��������������A�Ļ�ѧʽΪ______������A��������______��������B��______���ѧʽ����
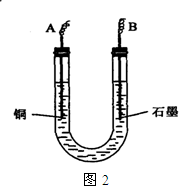
��2������������ò���������ͼ2��ʾ����AӦ��ֱ����Դ��______����B�缫�Ϸ����ĵ缫��ӦʽΪ______��
��3������ʵ���������Ҫ����______������ĸ����
A���������ǰ�缫������
B������缫�ں��ǰ������������ˮ��ϴ
C�����µ���缫�ϵ�ͭ������ϴ������
D���缫�ں�ɳ��صIJ����б��밴����ɡ����ء��ٺ�ɡ��ٳ��ؽ�������
E���ڿ����к�ɵ缫ʱ��������õ��º�ɷ�
��4����������Һ�м���ʯ����Һ���۲쵽��������______��
��5��ͭ�����ԭ�������ļ���ʽΪ______��
�⣺��1��pHΪ4��5�������£�Cu2+������ˮ�⣬��Fe3+������ȫˮ�⣬�����Թ�����CuO�����Եõ�����������ͭ��Һ���ʼ���CuOͨ������H+��������Һ��pHʹ֮���ߣ�ʹFe3+��ȫˮ���γ�Fe��OH��3��������ȥ��
�ʴ�Ϊ��CuO��������Һ��pH��4��5֮��ʹFe3+��ȫˮ�⣬Fe��OH��3��
��2���������Cu����������Cu������ӵ�Դ�ĸ�����ʯī�缫���ӵ�Դ����������A��B�ֱ���ֱ����Դ�ĸ�����������B�缫�Ϸ����ĵ缫��ӦʽΪ��4OH--4e-=O2��+2H2O
�ʴ�Ϊ������4OH--4e-=O2��+2H2O��
��3����Ҫȷ��������ͭ��������ͭ�缫ǰ������֮��Ϊ���ɵ�Cu��������Cu������ḽ�����ӣ�Ӧ��ϴ��ȥ������ͭ������������Ӧ���ڿ�����������£�Ӧ���º�ɣ���������֮��С����ƽ�ĸ�����˵����ɣ�����Ҫ����ABDE����������Ҫ����C������
�ʴ�Ϊ��A��B��D��E��
��4���������ͭ��Һ����Cu�����������ᣬ�������ӷ���ʽΪ��2Cu2++2H2O 2Cu��+O2��+4H+����Һ�����ԣ�����ʯ����Һ���ɫ��
2Cu��+O2��+4H+����Һ�����ԣ�����ʯ����Һ���ɫ��
�ʴ�Ϊ����Һ���ɫ��
��5��VmL���������ʵ���Ϊ =
= mol����Cu�����ԭ������ΪMr�����ݵ���ת���غ㣬��
mol����Cu�����ԭ������ΪMr�����ݵ���ת���غ㣬��
 ��2=
��2= mol��4�����Mr=
mol��4�����Mr= ��
��
�ʴ�Ϊ�� ��
��
��������1��pHΪ4��5�������£�Cu2+������ˮ�⣬��Fe3+������ȫˮ�⣬�����Թ�����CuO�����Եõ�����������ͭ��Һ���ʼ���CuO����pHֵ��Fe3+��ȫˮ���γ�Fe��OH��3��������ȥ��
��2���������ͭ����������Cu�缫���ӵ�Դ�ĸ�����ʯī�缫B���ӵ�Դ����������Һ������������ʧ���ӷ���������Ӧ��
��3����Ҫȷ��������ͭ��������ͭ�缫ǰ������֮��Ϊ���ɵ�Cu��������Cu������ḽ�����ӣ�Ӧ��ϴ��ȥ������ͭ������������Ӧ���ڿ�����������£�Ӧ���º�ɣ���������֮��С����ƽ�ĸ�����˵����ɣ�
��4���������ͭ��Һ����Cu�����������
��5�����ݵ���ת���غ����Cu�����ԭ��������
�����������ԲⶨCu�����ԭ������Ϊ���壬������ԭ�����Թ������̵����⡢���û�ѧ�����ѧ����ȣ��Ѷ��еȣ�����������ǽ���Ĺؼ�����Ҫѧ��������ʵ�Ļ������ۺ�����֪ʶ��������������������
�ʴ�Ϊ��CuO��������Һ��pH��4��5֮��ʹFe3+��ȫˮ�⣬Fe��OH��3��
��2���������Cu����������Cu������ӵ�Դ�ĸ�����ʯī�缫���ӵ�Դ����������A��B�ֱ���ֱ����Դ�ĸ�����������B�缫�Ϸ����ĵ缫��ӦʽΪ��4OH--4e-=O2��+2H2O
�ʴ�Ϊ������4OH--4e-=O2��+2H2O��
��3����Ҫȷ��������ͭ��������ͭ�缫ǰ������֮��Ϊ���ɵ�Cu��������Cu������ḽ�����ӣ�Ӧ��ϴ��ȥ������ͭ������������Ӧ���ڿ�����������£�Ӧ���º�ɣ���������֮��С����ƽ�ĸ�����˵����ɣ�����Ҫ����ABDE����������Ҫ����C������
�ʴ�Ϊ��A��B��D��E��
��4���������ͭ��Һ����Cu�����������ᣬ�������ӷ���ʽΪ��2Cu2++2H2O
 2Cu��+O2��+4H+����Һ�����ԣ�����ʯ����Һ���ɫ��
2Cu��+O2��+4H+����Һ�����ԣ�����ʯ����Һ���ɫ���ʴ�Ϊ����Һ���ɫ��
��5��VmL���������ʵ���Ϊ
 =
= mol����Cu�����ԭ������ΪMr�����ݵ���ת���غ㣬��
mol����Cu�����ԭ������ΪMr�����ݵ���ת���غ㣬�� ��2=
��2= mol��4�����Mr=
mol��4�����Mr= ��
���ʴ�Ϊ��
 ��
����������1��pHΪ4��5�������£�Cu2+������ˮ�⣬��Fe3+������ȫˮ�⣬�����Թ�����CuO�����Եõ�����������ͭ��Һ���ʼ���CuO����pHֵ��Fe3+��ȫˮ���γ�Fe��OH��3��������ȥ��
��2���������ͭ����������Cu�缫���ӵ�Դ�ĸ�����ʯī�缫B���ӵ�Դ����������Һ������������ʧ���ӷ���������Ӧ��
��3����Ҫȷ��������ͭ��������ͭ�缫ǰ������֮��Ϊ���ɵ�Cu��������Cu������ḽ�����ӣ�Ӧ��ϴ��ȥ������ͭ������������Ӧ���ڿ�����������£�Ӧ���º�ɣ���������֮��С����ƽ�ĸ�����˵����ɣ�
��4���������ͭ��Һ����Cu�����������
��5�����ݵ���ת���غ����Cu�����ԭ��������
�����������ԲⶨCu�����ԭ������Ϊ���壬������ԭ�����Թ������̵����⡢���û�ѧ�����ѧ����ȣ��Ѷ��еȣ�����������ǽ���Ĺؼ�����Ҫѧ��������ʵ�Ļ������ۺ�����֪ʶ��������������������

��ϰ��ϵ�д�
 �ܿ�����ĩ��̾�ϵ�д�
�ܿ�����ĩ��̾�ϵ�д�
�����Ŀ
 ��֪��pHΪ4��5����Һ�У�Cu2+������ˮ�⣬��Fe3+������ȫˮ�⣮ijѧ�����õ��CuSO4��Һ�ķ����ⶨͭ�����ԭ����������ͬѧ��pH=3.8�ữ�ġ�����Fe2��SO4��3���ʵ�CuSO4��Һ�м�������ĺ�ɫ��ĩX����ֽ������Һ����ͼ��ʾװ�õ�⣬����ij�缫����a g����һ�缫�ϲ�����״���µ�����VmL������˵����ȷ���ǣ�������
��֪��pHΪ4��5����Һ�У�Cu2+������ˮ�⣬��Fe3+������ȫˮ�⣮ijѧ�����õ��CuSO4��Һ�ķ����ⶨͭ�����ԭ����������ͬѧ��pH=3.8�ữ�ġ�����Fe2��SO4��3���ʵ�CuSO4��Һ�м�������ĺ�ɫ��ĩX����ֽ������Һ����ͼ��ʾװ�õ�⣬����ij�缫����a g����һ�缫�ϲ�����״���µ�����VmL������˵����ȷ���ǣ������� ��֪��pHΪ4��5����Һ�У�Cu2+������ˮ�⣬��Fe3+������ȫˮ�⣮ijͬѧ���õ������ͭ��Һ�ķ����ⶨͭ�����ԭ����������ͬѧ��pH=3.8�ĺ������������ʵ�����ͭ��Һ�м�������ĺ�ɫ��ĩX����ֽ������Һ����ͼ��ʾװ�õ�⣬����ij�缫����a g����һ�缫�ϲ�����״���µ�����V mL������˵����ȷ���ǣ�������
��֪��pHΪ4��5����Һ�У�Cu2+������ˮ�⣬��Fe3+������ȫˮ�⣮ijͬѧ���õ������ͭ��Һ�ķ����ⶨͭ�����ԭ����������ͬѧ��pH=3.8�ĺ������������ʵ�����ͭ��Һ�м�������ĺ�ɫ��ĩX����ֽ������Һ����ͼ��ʾװ�õ�⣬����ij�缫����a g����һ�缫�ϲ�����״���µ�����V mL������˵����ȷ���ǣ�������