��Ŀ����
�����£���
0.1 mol/L��HA��Һ��0.1 mol/L��NaOH��Һ�������Ϻ�(���Ի�Ϻ���Һ����ı仯)����û����Һ��pH����8���Իش��������⣺(1)�����Һ��pH����8��ԭ����(�����ӷ���ʽ��ʾ)________��
(2)�����Һ����ˮ�������c(H+)________0.1 mol/L��NaOH��Һ����ˮ�������c(H+)(����ڡ�����С�ڡ����ߡ����ڡ�)��
(3)��������Һ��������ʽ�ľ�ȷ������(���������)��
c(Na+)��c(A��)��________mol/L��
c(OH��)��c(HA)��________mol/L��
�𰸣�
������
������
|
������ ���� ���������� ���� |

��ϰ��ϵ�д�
�����Ŀ
|
�����йص������Һ���������ʵ���Ũ�ȹ�ϵ��ȷ���� | |
A�� |
�����£���0.1 mol��L��1��NH4Cl��Һ��0.05 mol��L��1��NaOH��Һ�������ϣ�c(Cl��)��c(Na+)��c(NH4+)��c(OH��)��c(H+) |
B�� |
�����£���CH3COOH��Һ�м���������NaOH���õ�pH��4�Ļ����Һ��c(Na+)��c(CH3COO��)��c(H+)��c(OH��) |
C�� |
Ũ�Ⱦ�Ϊ0.1 mol��L��1��Na2CO3��Һ��NaHCO3��Һ�������ϣ�c(Na+)��c(H+)��2c(CO32��)��c(OH��)��c(HCO3��) |
D�� |
�����£�pH��3��һԪ��HX��Һ��pH��11��һԪ��MOH��Һ�������ϣ�c(M+)��c(X��)��c(H+)��c(OH��) |
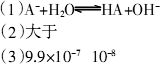

 CH3COO��+H+ ��H��0
CH3COO��+H+ ��H��0
