��Ŀ����
һ���������ܱ������У�4NH3(g)��5O2(g)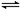 4NO(g)��6H2O(g)����H����905.9 kJ��mol��1��������������ȷ����
4NO(g)��6H2O(g)����H����905.9 kJ��mol��1��������������ȷ����
| A���ﵽƽ��ʱ�ų�����Ϊ905.9 kJ������ʼʱͶ��4 mol NH3��5 mol O2 |
| B��ƽ��ʱ4v��(O2)��5v��(NO) |
| C��ƽ���ѹ���������ƽ��Ħ��������С |
| D��NH3��O2��ʼʱ���ʵ���֮�ȵ���4:5����ƽ��������ʵ�ת����һ����� |
A
����

��ϰ��ϵ�д�
 ��ѧ�����ϵ�д�
��ѧ�����ϵ�д� �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
�����Ŀ
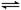 4NO(g)��6H2O(g)����H����905.9 kJ��mol��1��������������ȷ����
4NO(g)��6H2O(g)����H����905.9 kJ��mol��1��������������ȷ����