��Ŀ����
һ���¶ȷ�Χ�����Ȼ����۽��س�ʯ����Ҫ�ɷ�ΪKAlSi3O8�����Ƶ��Ȼ��أ���Ҫ��Ӧ�ǣ�NaCl��l����KAlSi3O8(s) KCl(l)��NaAlSi3O8(s)��
KCl(l)��NaAlSi3O8(s)��
��1���Ȼ��Ƶĵ���ʽ�� ��
��2��������Ӧ�漰��Ԫ���У�ԭ�Ӱ뾶������ ��
��3��������Ӧ�漰��λ��ͬһ���ڵļ���Ԫ���У���һ��Ԫ�ص�����������ˮ���������Ԫ�ص�����������ˮ������ܷ�����Ӧ����Ԫ���� ��
��4��ij��ȤС��Ϊ�о�������Ӧ�м�Ԫ�ص��۳��ʣ�Һ���м�Ԫ�ص�����ռ��Ʒ�����İٷ��ʣ����¶ȵĹ�ϵ������ʵ�飨���������������䣩������������ݣ�
| ʱ�䣨h�� �۳��� �¶� | 1.5 | 2.5 | 3.0 | 3.5 | 4.0 | 5.0 |
| 800�� | 0.054 | 0.091 | 0.127 | 0.149 | 0.165 | 0.183 |
| 830�� | 0.481 | 0.575 | 0.626 | 0.669 | 0.685 | 0.687 |
| 860�� | 0.515 | 0.624 | 0.671 | 0.690 | 0.689 | 0.690 |
| 950�� | 0.669 | 0.714 | 0.710 | 0.714 | 0.714 | �D�D |
�ٷ������ݿ��Եó����Ȼ����۽��س�ʯ�� ������ȡ������ȡ�����Ӧ��
��950��ʱ��������۳��ص����ʿ��Բ�ȡ�Ĵ�ʩ�� ������ţ���
a����ֽ���
b���ӳ���Ӧʱ��
c������Ӧ��ϵ��ѹǿ
d�����س�ʯ�۴�ɸ�С�Ŀ���
��5���÷����Ƶ�KCl�ᴿ�������ұ�������ء���ӦNa��l����KCl��l��
 NaCl��l����K��g���ǹ�ҵ��ұ�������س��õķ�������ƽ��ԭ�����÷������е�ԭ���� ��
NaCl��l����K��g���ǹ�ҵ��ұ�������س��õķ�������ƽ��ԭ�����÷������е�ԭ���� ��
��1�� ��2��K��3��Na��4�������� ��a d ��5�����ط�������������˲����Ũ�ȣ�ƽ�������ƶ���
��2��K��3��Na��4�������� ��a d ��5�����ط�������������˲����Ũ�ȣ�ƽ�������ƶ���
���������������1������д���Ȼ��Ƶĵ���ʽ�� ����2���Ƚ����Ӱ뾶���ٿ��㣬��Խ�࣬�뾶Խ��ͬ�㣬���ˣ��˵����Խ�뾶ԽС����ͬ�㡢ͬ�˿����ӣ�����Խ��뾶Խ��ԭ�Ӱ뾶������K����3��Ԫ���Ƶ�����������ˮ���������Ԫ�ص�����������ˮ������ܷ�����Ӧ����4���¶�Խ�ߣ���Ԫ�ص��۳���Խ���Ȼ����۽��س�ʯ�����ȹ��̣���950��ʱ��������۳��ص����ʿ��Բ�ȡ�Ĵ�ʩ�dz�ֽ��裬���س�ʯ�۴�ɸ�С�Ŀ������������ĽӴ��������5��������������ԭ�����ɲ�ȡ���ط�������������˲����Ũ�ȣ�ƽ�������ƶ���
����2���Ƚ����Ӱ뾶���ٿ��㣬��Խ�࣬�뾶Խ��ͬ�㣬���ˣ��˵����Խ�뾶ԽС����ͬ�㡢ͬ�˿����ӣ�����Խ��뾶Խ��ԭ�Ӱ뾶������K����3��Ԫ���Ƶ�����������ˮ���������Ԫ�ص�����������ˮ������ܷ�����Ӧ����4���¶�Խ�ߣ���Ԫ�ص��۳���Խ���Ȼ����۽��س�ʯ�����ȹ��̣���950��ʱ��������۳��ص����ʿ��Բ�ȡ�Ĵ�ʩ�dz�ֽ��裬���س�ʯ�۴�ɸ�С�Ŀ������������ĽӴ��������5��������������ԭ�����ɲ�ȡ���ط�������������˲����Ũ�ȣ�ƽ�������ƶ���
���㣺�������ʽ��д�����Ӱ뾶�ȽϺ�ƽ���ƶ�ԭ����

A��B��C��D��E���ֶ�����Ԫ�أ�ԭ���������������й���Ϣ���¡�
| Ԫ�� | �й���Ϣ |
| A | ����������Ӧ��ˮ���������⻯�ﷴӦ�������ӻ����� |
| B | �ؿ��к�������Ԫ�� |
| C | �����뱣����ú���� |
| D | ���ʼȿ������ᷴӦ���ֿ���NaOH��Һ��Ӧ |
| E | ԭ�������������ȴ�����������1�� |
��ش��������⣺
��1��A���⻯���ˮ��Һ��ʹ��̪��Һ��죬ԭ���ǣ��õ��뷽��ʽ��ʾ����ʵ������ȡ���⻯��Ļ�ѧ����ʽ�� ��
��2��A��B�����������Ϊ7��16����ԭ�ӷ��ӣ����л�������������ʵ��ŷ��йص��ǣ�����ţ���
������ ������ЧӦ �۳������ƻ� �ܹ⻯ѧ��Ⱦ
��3��C������������ˮ������E���ʷ�Ӧ��������������Һ�������ӷ���ʽ�� ��
��4��D��Ԫ�����ڱ��е�λ���ǡ���Ԫ��A��D���ij������������õ��Ե�ԣ���������ˮ������Ӧ�Ļ�ѧ����ʽ�� ��
����15N���Բⶨ�������ζԵ���ˮ�ʵ���Ⱦ�����
��1������˵����ȷ����___��
| A��14N��15NΪͬ�ֺ��� |
| B��14N�ĵ�һ������С��14C |
| C��15NH3��14NH3�����γɷ��Ӽ���� |
| D��CH315NH2��CH3CH214NH2��Ϊͬϵ�� |
��3�� ��ҵ�ϵ�ⷨ�����������ε�ģ�����ͼ��

��֪��������������ѧ��Ӧ��
2NO2-+8H++6Fe2+==N2�� +6Fe3++4HzO
�������缫��Ӧ����ʽ��______(�����Ǻ�����Ӧ����
����������ҺŨ�ȹ������������������ݳ���������Ϊ______(�ѧʽ��;���������c( H+ )Խ��H+������Խ______;
�������ʱ��·����0.6 mol����ת��,��NaNO2��ʣ��,�����������Na2SO4______mol��
X��Y��Z��W��Ԫ�����ڱ���ԭ������������������ֶ�����Ԫ�أ��������Ϣ���±���
| Ԫ�� | �����Ϣ |
| X | X������������Ӧ��ˮ���ﻯѧʽΪH2XO3 |
| Y | Y�ǵؿ��к�����ߵ�Ԫ�� |
| Z | Z�Ļ�̬ԭ�����������Ų�ʽΪ3s23p1 |
| W | W��һ�ֺ��ص�������Ϊ28��������Ϊ14 |
��1��Wλ��Ԫ�����ڱ��� ���ڵ� �壻W��ԭ�Ӱ뾶��X��
�����������
��2��Z�ĵ�һ�����ܱ�W�� �����С������ XY2�ɹ�̬��Ϊ��̬����˷��������������� ����Ԫ�ء�X��Y��ԭ�ӿɹ�ͬ�γɶ��ַ��ӣ�д������һ�����γ�ͬ�ַ��Ӽ�������������� ��
��3�����£���Z���������ᷴӦ�����ɫ��Һ�еμ�NaOH��Һֱ���������ܹ۲쵽�������� ��W�ĵ���������ᷴӦ����������ɫ���壬�÷�Ӧ�Ļ�ѧ����ʽ�� ��
��4����25�㡢101kpa�£���֪13.5g��Z���嵥����Y2��������ȫȼ�պ�ָ���ԭ״̬������419kJ���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��5����ҵ��ұ��Zʱ�õ��Ĵ����DZ���ʯ����ȡ����ʯ(Na3AlF6)�Ļ�ѧ����ʽ���£� 2Al(OH)3+ 12HF+ 3 A = 2Na3AlF6+ 3CO2��+ 9H2O
�����������������գ�
�ٷ�Ӧ��A�Ļ�ѧʽΪ ������ ����
�ڱ���ʯ��Na3AlF6�������ӻ���������������ɣ�����ʯ�����ṹ����ͼ��ʾ��
 λ�ڴ������嶥������ģ�
λ�ڴ������嶥������ģ� λ�ڴ��������12������е��8��С����������ģ���ô������������Ĵ��������������� �������������ţ�
λ�ڴ��������12������е��8��С����������ģ���ô������������Ĵ��������������� �������������ţ�
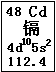
 Cd(OH)2�� 2Ni(OH)2
Cd(OH)2�� 2Ni(OH)2