��Ŀ����
����Ŀ��ӡ���ɽ�緢��������׳�۵����������������ľ���������ʲƸ������������ð������Σ���ڵײ��Ļ�ɽ���ռ�����ǿ���ȡ������롣��ǿ�������������ԭ�����ᡣij��������ͼ��ʾ�Ĺ��������������
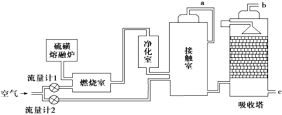
��ش��������⣺
��1��Ϊ������÷�Ӧ�ų����������Ӵ�����Ӧ��װ______________(���豸���ƣ��������������������ɹܣ���������_____________________________________________��
��2��Ϊʹ��dz��ȼ�գ���������1ͨ��ȼ���ҵ���������50%��Ϊ���SO2ת���ʣ���������2��������Ϊ�Ӵ����ж���������ȫ����ʱ������������2.5��������������������������1��������2�Ŀ��������ӦΪ________������Ӵ�����SO2��ת����Ϊ95%��b���ų���β���ж���������������Ϊ__________(���������������������0.2�ƣ�����β���Ĵ���������________��
��3������������Ϊԭ�ϵ�����������ȣ��ù��յ��ص���________(�ɶ�ѡ����
A������������ B�����������ת�������
C�������ķ������� D������Ҫʹ�ô���
��4���������;�dz��㣬��Ӧ����������Щ����__________________________��
A������ B��������Լ�������������ơ��ĺϳ�
C��Ǧ���ص����� D��������Ƶ��Ʊ�
��5������ȼ�ϵ�ȼ���Dz���������SO2����Ҫԭ��֮һ����ȼú�м���������ʯ��ʯ������Ч����úȼ��ʱSO2���ŷţ���д������������з�Ӧ�Ļ�ѧ����ʽ_____________��
���𰸡�
��1���Ƚ����� ʹŨH2SO4��SO3��ֽӴ�
��2��6��5 0.41% �ð�ˮ����
��3��A
��4��BCD
��5��CaCO3![]() CaO+CO2����SO2+CaO�TCaSO3��2CaSO3+O2�T2CaSO4
CaO+CO2����SO2+CaO�TCaSO3��2CaSO3+O2�T2CaSO4
��������
�����������1��SO2��O2�ķ�ӦΪ���ȷ�Ӧ��Ϊ�˳������������Ӧ��װ�Ƚ����������������������ɹܣ���������������Ũ����ĽӴ��棬���������������������
��2��ȼ�����еķ�ӦΪS+O2![]() SO2������SO2�����Ϊx����������1��ͨ�����������Ϊ1.5x���Ӵ����еķ�ӦΪ2SO2+O2
SO2������SO2�����Ϊx����������1��ͨ�����������Ϊ1.5x���Ӵ����еķ�ӦΪ2SO2+O2![]() 2SO3����������2��ͨ�����������Ϊ1.25x��������1��ͨ����������Ϊ7.5x��������2��ͨ����������Ϊ6.25x��������������1��������2�Ŀ��������ӦΪ7.5x��6.25x=6��5��ȼ����ʣ�����6.5x���Ӵ���ʣ�����6.25x-x=5.775x��ʣ��SO2Ϊ0.05x����b��β����SO2���������Ϊ0.41%��SO2Ϊ��������������ü�Һ���簱ˮ�����գ�
2SO3����������2��ͨ�����������Ϊ1.25x��������1��ͨ����������Ϊ7.5x��������2��ͨ����������Ϊ6.25x��������������1��������2�Ŀ��������ӦΪ7.5x��6.25x=6��5��ȼ����ʣ�����6.5x���Ӵ���ʣ�����6.25x-x=5.775x��ʣ��SO2Ϊ0.05x����b��β����SO2���������Ϊ0.41%��SO2Ϊ��������������ü�Һ���簱ˮ�����գ�
��3��������������������Ҫ����O2��A����ȷ��ԭ��ѡ����SO2��ת�����أ�B�������������Ϊԭ�ϲ����ķ����϶࣬����������ͬ��C�������SO2��ȡSO3�Ĺ����ж���Ҫʹ�ô�����D�������ѡA��
��4���������õ���Ϊ������A�����������������к��л��������ȡ��������Ҫ�����ǻ���Ӧ��B����ȷ��Ǧ��������Ҫ�õ����������Ǧ��C����ȷ��������Ƶ���ȡ��������ҪŨ�������ʯ��D����ȷ����ѡBCD��
��5��CaCO3���·ֽ�����CO2��CaO��SO2Ϊ������������Ժͼ���������CaO��Ӧ����CaSO3����CaSO3�ױ�����ΪCaSO4�����Է�����Ӧ�Ļ�ѧ����ʽΪ��CaCO3![]() CaO+CO2�� SO2+CaO�TCaSO3 2CaSO3+O2�T2CaSO4��
CaO+CO2�� SO2+CaO�TCaSO3 2CaSO3+O2�T2CaSO4��

����Ŀ��������(CH3OCH3)��һ��������Դ�����ö�����̼�ϳ�����Դ�ѳ�Ϊ������ѧ���о������ſ��⡣
��1�����ֹ��ۼ��ļ��������ʾ��
��ѧ�� | C=O | H-H | C-H | C-O | H-O |
����/kJ��mol-1 | 803 | 436 | 414 | 326 | 464 |
2CO2(g)+6H2(g)=CH3OCH3(g)+3H2O(g) ![]() _______________��
_______________��
��2�����˹�������á�����������Ŀ���ü���ģ������Ĺ�����ã�����̫���⽫H2O��CO2ֱ�Ӻϳ�ȼ�Ϻͻ���ԭ�ϡ�������Աģ�������ã������ͼ����ʾװ���Ʊ������ѡ�����ת����ʽΪ̫����![]() ����
����![]() ��ѧ�ܡ�
��ѧ�ܡ�
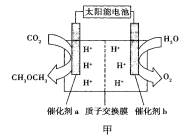
������b�ĵ缫������_________________��
�����ͷ�11.2L��������״���£�����___________mol H+___________�������ӵ�Ǩ�Ʒ���
������a�ϵĵ缫��ӦʽΪ______________��
��3����ʢ�ٴ�ѧ���о���Ա�о���һ�ַ�������ʵ��ˮ������ʱCO2�����ŷţ������ԭ����ͼ����ʾ��
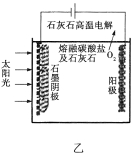
������������������ת����ʽ��__________________��
����ⷴӦ���¶�С��900![]() ʱ���У�̼��Ʒֽ�ΪCaO��CO2�������Ϊ����̼���ƣ��������ĵ缫��ӦʽΪ_______________�������ĵ缫��ӦʽΪ______________��
ʱ���У�̼��Ʒֽ�ΪCaO��CO2�������Ϊ����̼���ƣ��������ĵ缫��ӦʽΪ_______________�������ĵ缫��ӦʽΪ______________��







