��Ŀ����
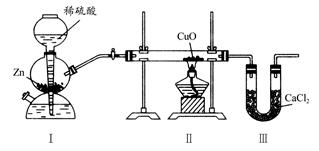
��ҵ���û���̿�������������ˮ�е�I-��ȡI2������������ͼ��
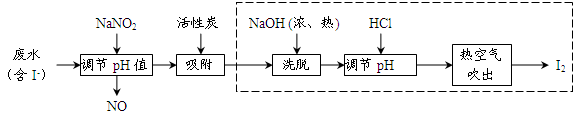
��1�����ˮ�м���NaNO2������pH<4��д����Ӧ�����ӷ���ʽ__________________��
��2���û���̿�������ɵĵ��ʵ��Ŀ����__________________________����Ũ����NaOHϴ�������ĵ�Ļ�ѧ����ʽΪ___________________________________��
��3���ӵ���pH�����õ���Һ�л�ȡ�⣬��ҵ�Ͽ����ȿ�����������ԭ����___________����ȡ��������Һ��ʵ�����з���õ����ʵ⣬��Ҫ���еIJ���Ϊ__________________��
��4�����������ɵ�NOβ���ж�����ҵ�Ͻ�����O2��һ������ͨ��NaOH�Ʊ�NaNO2��д����Ӧ�Ļ�ѧ����ʽ��________________________________��
��5����ҵ����������̻�������ͼ�������棬������ͼ���̵��ŵ�___________________________��д��һ������

��1�����ˮ�м���NaNO2������pH<4��д����Ӧ�����ӷ���ʽ__________________��
��2���û���̿�������ɵĵ��ʵ��Ŀ����__________________________����Ũ����NaOHϴ�������ĵ�Ļ�ѧ����ʽΪ___________________________________��
��3���ӵ���pH�����õ���Һ�л�ȡ�⣬��ҵ�Ͽ����ȿ�����������ԭ����___________����ȡ��������Һ��ʵ�����з���õ����ʵ⣬��Ҫ���еIJ���Ϊ__________________��
��4�����������ɵ�NOβ���ж�����ҵ�Ͻ�����O2��һ������ͨ��NaOH�Ʊ�NaNO2��д����Ӧ�Ļ�ѧ����ʽ��________________________________��
��5����ҵ����������̻�������ͼ�������棬������ͼ���̵��ŵ�___________________________��д��һ������

��1��2NO2-+ 4H++ 2I- �� 2NO + I2 + 2H2O ��2�֣�
��2���������ʵ⣨1�֣� 3I2 + 6NaOH �� NaIO3+ 5NaI+ 3H2O��2�֣�
��3�����ʵ���ˮ���ܽ��С����������1�֣� ��ȡ ��Һ ����3�֣�
��4��4NaOH+ O2+ 4NO �� 4NaNO2+ 2H2O��2�֣�
��5�������˻�ѧҩƷ��Ͷ������ʹ�ɱ���͡������������ڼ��٣���ҩƷ���豸��ʴ�Եȣ���1�֣��������ɣ�
��2���������ʵ⣨1�֣� 3I2 + 6NaOH �� NaIO3+ 5NaI+ 3H2O��2�֣�
��3�����ʵ���ˮ���ܽ��С����������1�֣� ��ȡ ��Һ ����3�֣�
��4��4NaOH+ O2+ 4NO �� 4NaNO2+ 2H2O��2�֣�
��5�������˻�ѧҩƷ��Ͷ������ʹ�ɱ���͡������������ڼ��٣���ҩƷ���豸��ʴ�Եȣ���1�֣��������ɣ�
�����������1����������ͼ�ż���NaNO2ʱ��NO���ɣ�������������ԭ��Ӧ���������ӷ���ʽ2NO2-+ 4H++ 2I- �� 2NO + I2 + 2H2O��
��2���û���̿�������ɵĵ��ʵ��Ŀ���Ǹ������ʵ⡣��������ͼ��������ʱ���е����ɣ���֪��Ũ����NaOHϴ�������ĵ�Ļ�ѧ����ʽΪ3I2 + 6NaOH �� NaIO3+ 5NaI+ 3H2O��
��3���ӵ���pH�����õ���Һ�л�ȡ�⣬��ҵ�Ͽ����ȿ�����������ԭ���ǵ��ʵ���ˮ���ܽ��С������������ȡ��������Һ��ʵ�����з���õ����ʵ��Ⱥ���еIJ���Ϊ ��ȡ����Һ������
��4������������ԭ��Ӧԭ������Ӧ�Ļ�ѧ����ʽ4NaOH+ O2+ 4NO �� 4NaNO2+ 2H2O��
��5������Դ�����úͻ�������˼�����𣬿����Ǽ����˻�ѧҩƷ��Ͷ������ʹ�ɱ���͡������������ڼ��٣���ҩƷ���豸��ʴ�Եȣ�

��ϰ��ϵ�д�
�����Ŀ