ĢāÄæÄŚČŻ
ŅŃÖŖNH4+ + OH£  NH3”ü + H2O£¬NH3ÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶”£ĻÖijČÜŅŗÖŠæÉÄÜŗ¬ÓŠĻĀĮŠ6ÖÖĄė×ÓÖŠµÄij¼øÖÖ£ŗNa+”¢NH4+”¢K+”¢Cl£”¢SO42£”¢CO32£”£ĪŖČ·ČĻČÜŅŗ×é³É½ųŠŠČēĻĀŹµŃé£ŗ£Ø1£©Č”200 mLÉĻŹöČÜŅŗ£¬¼ÓČė×ćĮæBaCl2ČÜŅŗ£¬·“Ó¦ŗ󽫳Įµķ¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ£¬µĆ³Įµķ4.30g£¬Ļņ³ĮµķÖŠ¼ÓČė¹żĮæµÄŃĪĖį£¬ÓŠ2.33g³Įµķ²»ČÜ”££Ø2£©Ļņ£Ø1£©µÄĀĖŅŗÖŠ¼ÓČė×ćĮæµÄNaOHČÜŅŗ£¬¼ÓČČ£¬²śÉśÄÜŹ¹ŹŖČóŗģÉ«ŹÆČļŹŌÖ½±äĄ¶µÄĘųĢå1.12L£ØŅŃ»»Ėć³É±ź×¼×“æö£¬¼Ł¶Ø²śÉśµÄĘųĢåČ«²æŅŻ³ö£©”£ÓÉ“ĖæÉŅŌµĆ³ö¹ŲÓŚŌČÜŅŗ×é³ÉµÄÕżČ·½įĀŪŹĒ
NH3”ü + H2O£¬NH3ÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶”£ĻÖijČÜŅŗÖŠæÉÄÜŗ¬ÓŠĻĀĮŠ6ÖÖĄė×ÓÖŠµÄij¼øÖÖ£ŗNa+”¢NH4+”¢K+”¢Cl£”¢SO42£”¢CO32£”£ĪŖČ·ČĻČÜŅŗ×é³É½ųŠŠČēĻĀŹµŃé£ŗ£Ø1£©Č”200 mLÉĻŹöČÜŅŗ£¬¼ÓČė×ćĮæBaCl2ČÜŅŗ£¬·“Ó¦ŗ󽫳Įµķ¹żĀĖ”¢Ļ“µÓ”¢øÉŌļ£¬µĆ³Įµķ4.30g£¬Ļņ³ĮµķÖŠ¼ÓČė¹żĮæµÄŃĪĖį£¬ÓŠ2.33g³Įµķ²»ČÜ”££Ø2£©Ļņ£Ø1£©µÄĀĖŅŗÖŠ¼ÓČė×ćĮæµÄNaOHČÜŅŗ£¬¼ÓČČ£¬²śÉśÄÜŹ¹ŹŖČóŗģÉ«ŹÆČļŹŌÖ½±äĄ¶µÄĘųĢå1.12L£ØŅŃ»»Ėć³É±ź×¼×“æö£¬¼Ł¶Ø²śÉśµÄĘųĢåČ«²æŅŻ³ö£©”£ÓÉ“ĖæÉŅŌµĆ³ö¹ŲÓŚŌČÜŅŗ×é³ÉµÄÕżČ·½įĀŪŹĒ
A”¢Ņ»¶Ø“ęŌŚSO42£”¢CO32£”¢NH4+£¬æÉÄÜ“ęŌŚNa+”¢K+”¢Cl£
B”¢Ņ»¶Ø“ęŌŚSO42£”¢CO32£”¢NH4+”¢Cl££¬Ņ»¶Ø²»“ęŌŚNa+”¢K+
C”¢c(CO32£)=0.01 mol/L£¬c(NH4+)£¾c(SO42£)
D”¢Čē¹ūÉĻŹö6ÖÖĄė×Ó¶¼“ęŌŚ£¬Ōņc(Cl£)£¾c(SO42£)
D
”¾½āĪö”æ
ŹŌĢā·ÖĪö£ŗȔɣĮæøĆČÜŅŗ¼ÓČėBaCl2ČÜŅŗÓŠ°×É«³ĮµķÉś³É£¬ŌŁ¼ÓČė×ćĮæŃĪĖįŗ󣬳Įµķ²æ·ÖČܽā£¬²¢ÓŠĘųĢåÉś³É£¬ĖµĆ÷°×É«³ĮµķĪŖBaCO3ŗĶBaSO4£¬ŌņČÜŅŗÖŠŗ¬ÓŠCO32-”¢SO42-£»Ļņ£Ø1£©µÄĀĖŅŗÖŠ¼ÓČė×ćĮæµÄNaOHČÜŅŗ£¬¼ÓČČ£¬²śÉśÄÜŹ¹ŹŖČóŗģÉ«ŹÆČļŹŌÖ½±äĄ¶µÄĘųĢ壬ĖµĆ÷ČÜŅŗÖŠÓŠNH4+£»ĮķČ”ÉĻŹöŌČÜŅŗÉŁĮ棬µĪ¼ÓÓĆĻõĖįĖį»ÆµÄĻõĖįŅųČÜŅŗ£¬Ć»ÓŠĆ÷ĻŌĻÖĻó£¬ĖµĆ÷ČÜŅŗ֊ƻӊCl-£®¹ŹČÜŅŗÖŠŅ»¶Ø“ęŌŚSO42-”¢CO32-”¢NH4+£¬Ņ»¶Ø²»“ęŌŚCl-£¬æÉÄÜ“ęŌŚNa+”¢K+”£³Įµķ4.30gĪŖBaCO3ŗĶBaSO4µÄ×ÜĮ棬ÓÖĻņ³ĮµķÖŠ¼ÓČė¹żĮæµÄŃĪĖį£¬ÓŠ2.33g³Įµķ²»ČÜ£¬¹ŹBaSO4µÄĪļÖŹµÄĮæĪŖ(2.33g)/(233g/mol)=0.01mol, ŌņBaCO3µÄµÄĪļÖŹµÄĮæĪŖ0.01 mol£¬c(CO32£)=0.01mol/0.2L=0.05 mol/L,ÓÖNH4+ + OH£  NH3”ü + H2O£¬ĒŅNH3µÄĪļÖŹµÄĮæĪŖ1.12L/(22.4L/mol)=0.05mol”£Ōņc(NH4+)£¾c(SO42£)”£Čē¹ūÉĻŹö6ÖÖĄė×Ó¶¼“ęŌŚ£¬Ōņ“ęŌŚČēĻĀĘ½ŗā£ŗn(Na+)+ n(K+)+ n(NH4+)= n(SO42£) +n (CO32£) +n (Cl£)£¬¼“n(Na+)+ n(K+)+0.05mol=0.01mol+0.01mol+ n (Cl£),¹Źn (Cl£)=0.03+ n(Na+)+ n(K+)©n(SO42£) ¼“c(Cl£)£¾c(SO42£)”£
NH3”ü + H2O£¬ĒŅNH3µÄĪļÖŹµÄĮæĪŖ1.12L/(22.4L/mol)=0.05mol”£Ōņc(NH4+)£¾c(SO42£)”£Čē¹ūÉĻŹö6ÖÖĄė×Ó¶¼“ęŌŚ£¬Ōņ“ęŌŚČēĻĀĘ½ŗā£ŗn(Na+)+ n(K+)+ n(NH4+)= n(SO42£) +n (CO32£) +n (Cl£)£¬¼“n(Na+)+ n(K+)+0.05mol=0.01mol+0.01mol+ n (Cl£),¹Źn (Cl£)=0.03+ n(Na+)+ n(K+)©n(SO42£) ¼“c(Cl£)£¾c(SO42£)”£
æ¼µć£ŗ³£¼ūŃōĄė×ӵļģŃ飻³£¼ūŅõĄė×ӵļģŃ锣

 ŠÄĖćæŚĖćĒÉĖćŅ»æĪŅ»Į·ĻµĮŠ“š°ø
ŠÄĖćæŚĖćĒÉĖćŅ»æĪŅ»Į·ĻµĮŠ“š°ø Ó¦ÓĆĢā×÷Ņµ±¾ĻµĮŠ“š°ø
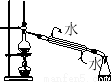
Ó¦ÓĆĢā×÷Ņµ±¾ĻµĮŠ“š°øČēĶ¼ŹĒ²ā¶ØĀĮ·Ū£Øŗ¬Ć¾·Ū£©µÄ“æ¶ČµÄŹµŃé×°ÖĆ”£ĖłÓƵÄNaOH£Ø×ćĮ棩µÄĪļÖŹµÄĮæÅضČĪŖ4.5 mol”¤L£1”£²»Ķ¬Ź±¼äµē×ÓĢģĘ½µÄ¶ĮŹżČēĻĀ±ķĖłŹ¾£ŗ
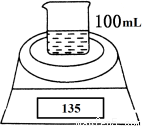
ŹµŃé²Ł×÷ | Ź±¼ä/min | µē×ÓĢģĘ½µÄ¶ĮŹż/g |
ÉÕ±£«NaOHČÜŅŗ | 0 | 120 |
ÉÕ±£«NaOHČÜŅŗ£«ŃłĘ· | 0 | 135 |
1 | 134.5 | |
2 | 134.1 | |
3 | 133.8 | |
4 | 133.8 |
£Ø1£©·“Ó¦ÖŠÉś³ÉĘųĢåµÄÖŹĮæ g”£
£Ø2£©ŹŌ¼ĘĖćѳʷ֊ĀĮµÄÖŹĮæ·ÖŹż”£(Š“³ö½āĢā¹ż³Ģ)
£Ø3£©·“Ó¦ŗóČÜŅŗ(ČÜŅŗµÄĢå»ż±ä»ÆŗöĀŌ)µÄc(OH£)”£(Š“³ö½āĢā¹ż³Ģ)