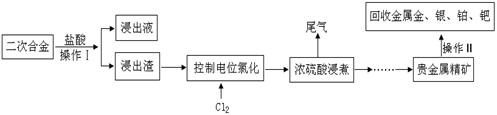
��Ŀ����
���κϽ��к��н����������ٵȹ��ؽ�����ʵ�����Զ��κϽ�Ϊԭ����ȡ�����������ٵȹ��ؽ����IJ������£��Իش��������⣺
��1��Ũ�����������в���β������Ҫ�ɷ��� ����д���ţ���ͬ�������Ը�������м��飬��õ��Լ��� ��
��2���������ʵ�������� ����ɸ�ʵ����Ҫ�IJ��������� ����д�������ƣ���
��3��ʵ�����Ʊ���Cl2�л���ˮ������HCl���ʣ���ͬѧ���������ͼ��ʾ��ʵ��װ����֤��ˮ������HCl���ʵĴ��ڣ�����ݼ�ͬѧ�������ͼ��������й����⡣
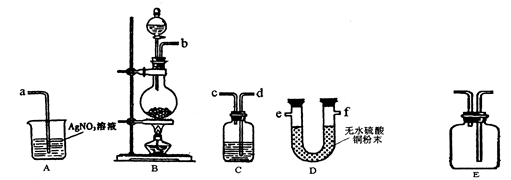
�ٸ�ʵ��װ�õĽӿ�˳��Ϊ��b�� �� �� �� ��a��
��װ��C�������� ��
����ͬѧ��Ϊ��ͬѧ��ʵ����ȱ�ݣ�����֤������ͨ��AgNO3��Һ�е�����ֻ��һ�֡�Ϊ��ȷ��ʵ����۵Ŀɿ��ԣ�֤������ͨ��AgNO3��Һ������ֻ��һ�֣���ͬѧ�����ij����װ��֮���ټ�װ��E������Ϊװ��EӦ����
���� ֮�䣨��װ����ţ��������ڹ��ƿ�з���
����д�����Լ�����Ʒ���ƣ���

��1��Ũ�����������в���β������Ҫ�ɷ��� ����д���ţ���ͬ�������Ը�������м��飬��õ��Լ��� ��
| A��H2 | B��SO2 | C�����ȵ�����ͭ��ĩ | D��Ʒ���Լ� |
��3��ʵ�����Ʊ���Cl2�л���ˮ������HCl���ʣ���ͬѧ���������ͼ��ʾ��ʵ��װ����֤��ˮ������HCl���ʵĴ��ڣ�����ݼ�ͬѧ�������ͼ��������й����⡣

�ٸ�ʵ��װ�õĽӿ�˳��Ϊ��b�� �� �� �� ��a��
��װ��C�������� ��
����ͬѧ��Ϊ��ͬѧ��ʵ����ȱ�ݣ�����֤������ͨ��AgNO3��Һ�е�����ֻ��һ�֡�Ϊ��ȷ��ʵ����۵Ŀɿ��ԣ�֤������ͨ��AgNO3��Һ������ֻ��һ�֣���ͬѧ�����ij����װ��֮���ټ�װ��E������Ϊװ��EӦ����
���� ֮�䣨��װ����ţ��������ڹ��ƿ�з���
����д�����Լ�����Ʒ���ƣ���
����13�֣���1��B�� D������2�֣���2�����ˡ���1�֣��� ��ͨ©�������������ձ�����2�֣�
��3����b��e��f��d��c��a��b��f��e��d��c��a������2�֣�������Cl2������2�֣�
��AC��2�֣���ʪ���KI������ֽ������ʪ����ɫ����������2�֣�
��3����b��e��f��d��c��a��b��f��e��d��c��a������2�֣�������Cl2������2�֣�
��AC��2�֣���ʪ���KI������ֽ������ʪ����ɫ����������2�֣�
�����������1��Ũ�������ǿ�����ԣ��ܰѽ��������������ᱻ��ԭΪSO2������β������Ҫ�ɷ���SO2��SO2����Ư���ԣ���ʹƷ����Һ��ɫ�����Լ�����Լ���Ʒ����Һ��
��2���ѹ������Һ�з�����IJ����ǹ��ˣ���ɸ�ʵ����Ҫ�IJ�����������ͨ©�������������ձ���
��3�������������������Ȼ��⣬��ˮ����ͭ��������ˮ�������������Ȼ���ͨ����������Һ�У������ˮ�������������ȼ���ˮ����������Ϊ����Ҳ�ܺ���������Ӧ���ɰ�ɫ�����Ȼ����������ȷ��˳����b��e��f��d��c��a��b��f��e��d��c��a��
�ڸ��ݢٿ�֪��װ��C��������������������ֹ�����Ȼ���ļ��顣
�����ڲ���ȷ��Cװ���Ƿ���ȫ���������գ������Ҫ��ACװ��֮������1�����������Ƿ���ȫ���յ�װ�á����������������Կ�֪��ѡ����Լ�������ʪ���KI������ֽ������ʪ����ɫ��������
��������ѧ��һ����ʵ��Ϊ������ѧ�ƣ������л�ѧʵ�鼴��ѧ̽��֮˵�����ݹ۽�����߿�����Ҫ�Կ���̽����ʵ��������Ʊ�ʵ��Ϊ������Щ̽���Ժ��Ʊ���ʵ������⣬�ۺ���ǿ�����ۺ�ʵ������ϵ���ܣ��еĻ��ṩһЩ�µ���Ϣ�����Ҫ��ѧ���������桢ϸ�µ����⣬��ϵ��ѧ����֪ʶ�ͼ��ܣ�����֪ʶ����ȡ�Ǩ�ơ����飬ȫ��ϸ�µ�˼�����ܵó���ȷ�Ľ��ۡ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ







 �ٹ����Լ� �ڷ������NaCl�͵ⵥ�� ��ϡ��Ũ���� �ܶ���
�ٹ����Լ� �ڷ������NaCl�͵ⵥ�� ��ϡ��Ũ���� �ܶ���