��Ŀ����
ʵ������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���Ũ���ᷴӦ���������Ķ����������������ͼ��ʾʵ����ȷ�������������������ϩ�Ͷ��������Իش���������

��1��ʵ�����Ʊ���ϩ�Ļ�ѧ����ʽ �����µ�140��ʱ�ĸ���Ӧ����Ľṹ��ʽ
��2��ͼ�Т١��ڡ��ۡ���װ�ÿ�ʢ�ŵ��Լ��ǣ��� ���� ���� ���� ��(�������й��Լ����������ո���)
A��Ʒ����Һ B��NaOH��Һ C��Ũ���� D������KMn04��Һ
��3����˵����������������ڵ�������
��4��ʹ��װ�âڵ�Ŀ����
��5��ʹ��װ�â۵�Ŀ���� ��
��6��ȷ��������ϩ��������
(11��) ����ʽ2�� ����1��
(1)CH3CH2OH![]() CH2��CH2����H2O CH3CH2��O��CH2CH3
CH2��CH2����H2O CH3CH2��O��CH2CH3
��2���� A ���� B ���� A ���� D ��
��3��װ�â���Ʒ����Һ��ɫ��4�����������������������ϩ������ʵ��
��5��������������Ƿ���� ��6���� �е���Һ��ɫ�����е���Һ����ɫ

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д� ʵ������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ���������Ķ������������������ʵ����ȷ�������������������ϩ�Ͷ�������
ʵ������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ���������Ķ������������������ʵ����ȷ�������������������ϩ�Ͷ�������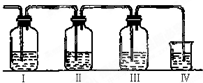 ʵ������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ���������Ķ������������������ʵ����ȷ�������������������ϩ�Ͷ�������I����װ�ÿ�ʢ�ŵ��Լ��ֱ��ǣ�I��Ʒ����Һ��NaOH��Һ��Ʒ����Һ��������KMnO4
ʵ������ȡ��ϩ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ���������Ķ������������������ʵ����ȷ�������������������ϩ�Ͷ�������I����װ�ÿ�ʢ�ŵ��Լ��ֱ��ǣ�I��Ʒ����Һ��NaOH��Һ��Ʒ����Һ��������KMnO4
