��Ŀ����
����Ŀ����ȩ(HCHO)�׳���ȩ����һ����Ҫ�Ļ���ԭ�ϡ���ͨ�����·������״�ת��Ϊ��ȩ��
���ⷨ��CH3OH(g)=HCHO(g)��H2(g) ��H1����92.09 kJ��mol��1
��������CH3OH(g)��![]() O2(g)=HCHO(g)��H2O(g)��H2
O2(g)=HCHO(g)��H2O(g)��H2
�ش���������:
(1)��֪��2H2(g)��O2(g)=2H2O(g)��H3����483.64 kJ��mol��1������H2��_________________��
(2)�����ⷨ��ȣ�������������ѧ�����ƽϴ���ԭ��Ϊ________________________________________��
(3)ͼ1Ϊ�״��Ʊ���ȩ��Ӧ��lg K(KΪƽ�ⳣ��)���¶�(T)�ı仯���ߡ�����_____(����a������b��)��Ӧ���ⷨ���ж�������_____________________________________��


(4)����ȩˮ��Һ�백ˮ����������Ƶ�������Ʒ(�ṹ��ʽ��ͼ2)����������ҽҩ�ȹ�ҵ���й㷺��;����ԭ����ȫ��Ӧ����������Ʒ�����ȩ�백�����ʵ���֮��Ϊ___________��
(5)���ڼ�ȩ�����Σ�����彡����ͨ�����������Լ������м�ȩ�ĺ�����һ��ȼ�ϵ���ͼ�ȩ���崫������ԭ����ͼ3��ʾ����a���ĵ缫��ӦʽΪ_________________________________________________������·��ת��4��10��4 mol����ʱ���������ڲμӷ�Ӧ��HCHOΪ________________mg��
���𰸡���149.73 kJ��mol��1 ���ⷨ�ķ�ӦΪ���ȷ�Ӧ���������ķ�ӦΪ���ȷ�Ӧ�����ȷ�Ӧ������ѧ�����ƽϴ� b ���ⷨΪ���ȷ�Ӧ���¶����ߣ�K���� 3��2 HCHO��H2O -4e�� = CO2��4H�� 3
��������
(1)��֪i��CH3OH(g)�THCHO(g)+H2(g)��H1=+92.09kJmol-1
ii.2H2(g)+O2(g)�T2H2O(g)��H3=-483.64kJmol-1��
���ݸ�˹����i+![]() ii�÷���ʽCH3OH(g)+
ii�÷���ʽCH3OH(g)+![]() O2(g)=HCHO(g)+H2O(g)��H2=(+92.09-
O2(g)=HCHO(g)+H2O(g)��H2=(+92.09-![]() ��483.64)kJ/mol=-149.73kJmol-1��
��483.64)kJ/mol=-149.73kJmol-1��
(2)�����Ȼ�ѧ��Ӧ����ʽ��֪���ⷨ�ʱ������Ϊ���ȷ�Ӧ���������ʱ�С����Ϊ���ȷ�Ӧ�����ȷ�Ӧ������ѧ�����ƽϴ�
(3)���ⷨΪ���ȷ�Ӧ���¶����ߣ�K������������b��Ӧ�������ⷨ��
(4)����ȩˮ��Һ�백ˮ����������Ƶ�������Ʒ����ԭ����ȫ��Ӧ����������Ʒ��ÿ��������Ʒ�����к���6��Cԭ�ӡ�4��Nԭ�ӣ�ÿ����ȩ�����к���1��Cԭ�ӡ�ÿ�����������к���1��Nԭ�ӣ�����Cԭ�ӡ�Nԭ���غ�֪��Ҫ�γ�һ��������Ʒ������Ҫ6����ȩ���ӡ�4���������ӣ�����Ҫ��ȩ�Ͱ������Ӹ���֮��=6��4=3��2�������ʵ���֮��Ϊ3:2��
(5)��ͼ��֪a��HCHO����������CO2����aΪԭ��ظ�����ʧ���ӷ���������Ӧ���缫����ʽΪHCHO��H2O -4e�� = CO2��4H�������ݵ缫����ʽ��֪ת��4��10��4 mol����ʱ�����뷴Ӧ��HCHOΪ1��10��4mol������Ϊ1��10��4mol��30g/mol=0.003g=3mg��

����Ŀ������ʵ���У��ܴﵽ��Ӧʵ��Ŀ�ĵ���
|
|
|
|
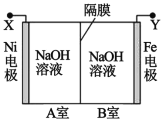
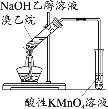
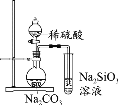
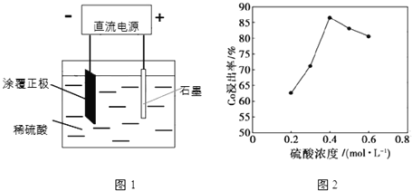
A���Ʊ����ռ��������� | B��֤���Ȼ����ܽ�ȴ������� | C����֤���������ȥ��������ϩ | D���ƶ�S��C��Si�ķǽ�����ǿ�� |
A.AB.BC.CD.D