��Ŀ����
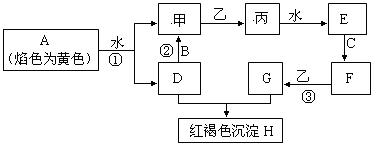
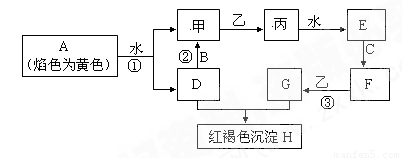
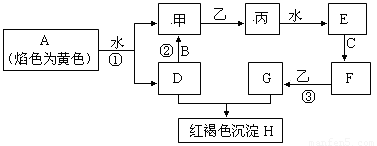
��14�֣� ��ͼ�������У�A��B��C�dz����������ʣ��ס��ҡ�����������������������ɫ�����壨ͼ����Щ��Ӧ�IJ���ͷ�Ӧ������û��ȫ���������
�����������Ϣ�ش��������⣺
��1��д����H�Ļ�ѧʽ_________���ҵ�������Ҫ��;________________________________
��2��д���йط�Ӧ�����ӷ���ʽ��
��Ӧ��__________________________________________________________
��Ӧ��__________________________________________________________
��3���жϷ�Ӧ�ۼ��������ij����Լ���______________________
��4����̬�����壨Һ�ܽ�����ҽѧ������Ҫ��;��C���ʶ�ӦԪ�ص�ij�������������Ǵ������е���Ҫ���ӣ��ô��������Ҫ�Ʊ��������£�����NH3ͨ��F��G�Ļ����Һ��F��G���ʵ�����Ϊ1��2���л��������ּ���������ּ�ǡ�÷�Ӧ�͵õ��������д�������з�Ӧ�Ļ�ѧ����ʽΪ��_________________________��ȷ���ô����强�ɹ���ȡ�ķ���_________________________________
��1��Fe��OH��3���������ᡢƯ����ũҩ���ϳ���ά������ɱ����
��2��2Na+2H2O=2Na++2OH��+H2����2Al+2OH��+2H2O=2AlO2��+3H2��
��3�� KSCN�����������Ĵ�Ҳ�ԣ�
��4��Fe��OH��2 + 2Fe��OH��3=Fe3O4 + 4H2O���������ʵ�飬�ܲ����������������������Ҳ�ԣ���
��������

 ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д� ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�