��Ŀ����
������Һ������˵����ȷ���ǣ� ��
| A��c(H+)�Uc(OH��)��1:10-2����Һ��K+��Ba2+��ClO����CO32��һ���ܴ������� |
| B��ˮ���������c(H+)��10-13mol/L����Һ��K+��Cl����NO3����I��һ���ܴ������� |
C����0.1mol/LCH3COOH��Һ��ͨ������HCl������ĵ���ƽ�����淴Ӧ�����ƶ�������Һ��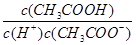 ���� ���� |
| D�������ʵ���Ũ�ȵ�������Һ����H2CO3 ��Na2CO3 ��NaHCO3 |
D
�������������A���ɸ�������ȷ����ҺΪ���ԣ�ClO����CO32�����ܴ������ڣ�B����Һ����Ϊ���Ի���ԣ���Ϊ����ʱ��I����������������C��ͨ��HCl����Һ�е�c��H+������ֵ��С������D��(NH4)2CO3��������˫ˮ�⣬c(CO32��)С��Na2CO3 �� H2CO3 �ĵڶ�������̫��������c(CO32��)С��NaHCO3��
���㣺���ӹ��桢������ʵĵ���ƽ�⡢����Ũ�ȴ�С�ıȽϡ�

��ϰ��ϵ�д�
�����Ŀ
�����й�˵����ȷ����
A�����д���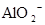 ����Һ�У�K���� ����Һ�У�K����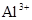 �� �� �� ��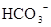 �ɹ��� �ɹ��� |
B��CO��g����ȼ������283.0kJ��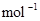 ���� ����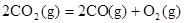 ��Ӧ�� ��Ӧ��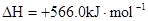 |
| C����NaOH��Һ��̹��ղ����ϵ����� |
D����ij������Һ�к��е����ʵ����� �� �� �� �� �� �� �� ��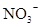 �� �� ��һ�ֻ���֣����������������������ݣ���Һ��ɫ����Գ��壬��������������3�֣���ԭ��Һ��һ���� ��һ�ֻ���֣����������������������ݣ���Һ��ɫ����Գ��壬��������������3�֣���ԭ��Һ��һ���� |
�������ӷ���ʽ��ȷ����( )
| A��������CO2ͨ�뱥��̼������Һ��: CO2��CO32����H2O��2HCO3�� |
B��FeSO4��Һ�ڿ����б��ʣ�4Fe2+��O2��2H2O 4Fe3+��4OH�� 4Fe3+��4OH�� |
| C�����������Һ��ͨ��������������Ca2+��ClO-��SO2��H2O��CaSO4����Cl-��2H�� |
| D��̼����þ��Һ�м������ʯ��ˮ Mg2++2HCO3��+2Ca2++4OH�� ®2CaCO3��+ Mg(OH)2��+ 2H2O |
���и����������������Һ�еķ�Ӧ������ͬһ���ӷ���ʽ��ʾ����
| A�����������������������������ͭ |
| B��BaCl2��Һ��Na2SO4��Һ��Ba(OH)2��Һ��H2SO4��Һ |
| C��Na2CO3��Һ��������Һ��CaCO3��Һ��������Һ |
| D��ʯ��ʯ�����ᷴӦ��ʯ��ʯ������ |
��pH��13����ɫ��Һ�У����и��������ܴ����������
A��K����Na���� �� �� | B��Na���� �� �� ��Cl�� ��Cl�� |
C��K����Na���� ��Br�� ��Br�� | D��K����Cu2����Cl���� |
����˵����ȷ����(�� ��)
| A��BaSO4��ˮ�к��ѵ��磬����BaSO4�Ƿǵ���� |
| B����������ˮ�õ��İ�ˮ�ܵ��磬����ˮ�ǵ���� |
| C����̬�����ӻ����ﲻ���磬����̬�����ӻ������ܵ��� |
| D��ǿ�������Һ�ĵ�������һ�������������Һ�ĵ�������ǿ |
�����£����и���������ָ����Һ�п��ܴ����������
| A��0.1mol/LNaHSO4��Һ�У� K+ NH4+ HCO3����NO3���� |
| B��������Ӧ������������Һ�У�Na+ NH4+ SO42���� CH3COO�� |
| C���μӷ�̪�Ժ�ɫ����Һ�У� Na+��NO3����SO42����CO32�� |
| D��NaNO3��Һ�У� Fe2+��H+�� Cl����I�� |
 NH3��+Al(OH)3��+2BaSO4��+H2O
NH3��+Al(OH)3��+2BaSO4��+H2O