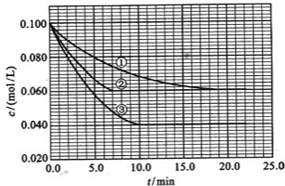
��Ŀ����
������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȡ�
|
|
Q |
R |
|
|
T |
|
|
W |
��1��T��ԭ�ӽṹʾ��ͼ��______���û�ѧ����ʽ��ʾ��ҵ����ұ��T���ʵ�ԭ����______��
��2����Wͬ�����ijԪ�أ����⻯������к���18�����ӣ��÷����д��ڵĹ� �ۼ���������______��
��3��Ԫ�صķǽ����ԣ�Q______W(�ǿ�ڡ������ڡ�)����Ϸ���ʽ������ԭ����______��
��4������R�������ͨ��״���³ʺ���ɫ������һ�Թܼף���ʹԪ��Rȫ��ת��Ϊ������������Ӧˮ���ʵ�鲽�裺��ʢ�м��Թܵ�����ˮ���У�______��
��1�� 2Al2O3
2Al2O3  4Al+3O2��
4Al+3O2��
��2�����Լ��ͷǼ��Լ�
��3������ CO32����H2O HCO3����OH�� ��̼���ˮ�⣬�������������ˮ��
HCO3����OH�� ��̼���ˮ�⣬�������������ˮ��
��4�����Թ��л���ͨ����������
��������
����������ɶ�����Ԫ�ؼ�ͼ��֪����Q��R�ڵڶ����ڣ�T��������������������������ȣ���T�ڵ������ڵڢ�A�壬��TΪAl�������Ƴ�QΪC��RΪN��WΪS��
��1��TΪAl��ԭ�ӽṹ����3�����Ӳ㣬����������Ϊ3��ԭ�ӽṹʾ��ͼΪ
��2����Wͬ�����ijԪ�أ����⻯������к���18�����ӣ��÷���ΪH2O2���÷��Ӵ��ڵĻ�ѧ������Ϊ�����Լ��ͷǼ��Լ���
��3������Na2CO3��Һ�����ԣ�Na2SO4��ҺΪ���ԣ�̼�ķǽ�����������ķǽ����ԣ�CO32‾ˮ�ⷴӦ���ӷ���ʽΪ��CO32����H2O HCO3����OH�� ��̼���ˮ�⣬�������������ˮ�⡣
HCO3����OH�� ��̼���ˮ�⣬�������������ˮ�⡣
��4������R�������ͨ��״���³ʺ���ɫ��ΪNO2�����Թ��л���ͨ������������������Ӧ��4NO2+O2+2H2O=4HNO3����ȫ��ת��ΪHNO3��
���㣺���⿼��Ԫ�ص��ƶϡ���ѧ�������ԵıȽϡ����ʵ����ʡ�

 ������������ϵ�д�
������������ϵ�д�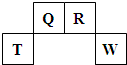 ��2009?������������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�
��2009?������������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�
 ������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�
������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ�
 ������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ������ͼ��ʾ������Ԫ��T��������������������������ȣ���ش��������⣺
������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ������ͼ��ʾ������Ԫ��T��������������������������ȣ���ش��������⣺
 ��2013?��خ��ģ�⣩������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ������ƶ���ȷ���ǣ�������
��2013?��خ��ģ�⣩������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ������ƶ���ȷ���ǣ�������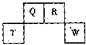 ��2012?������һģ��������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ���ش��������⣺
��2012?������һģ��������Ԫ��Q��R��T��W��Ԫ�����ڱ��е�λ����ͼ��ʾ������T��������������������������ȣ���ش��������⣺