��Ŀ����
6����ͼ1��ʾ��������ʯ���ͣ�17��̼���ϵ�Һ̬����������ʯ����Ӳ�ʲ����ܵĵײ����Թ��м������Ƭ�������Ƭ��ǿ�ȣ�ʯ��������ͨ�����ȵ����Ƭ���森��1���������ɵ�����ͨ�����Ը��������Һ�����Ը��������Һ��ɫ����ɫ���Һ���Ϊ���㣬�ϲ�Ϊ��ɫ��״Һ���²���ɫˮ״Һ�壮
��2���������ɵ�����ͨ����ˮ����ˮҲ��ɫ��

��ش��������⣺
��1��ʵ��װ���п�����������ʯ���͵����壬���Ƭ�������Ǵ�����
��2�����Ը��������Һ��ɫ���л���������Ļ�ѧ��Ӧ��������������Ӧ��
��3����ˮ��ɫ���л���������Ļ�ѧ��Ӧ�������Ǽӳɷ�Ӧ�����ܹ۲쵽������������Һ��ֳ�����
�ϲ���²㶼����ɫ����״���м�һ���ˮ״��
��4���������ɵ�������ͼ2��ʾ��װ�ý����ռ������飬����ˮ�е��Թ����γɵ���״Һ�壬��һ�Թ��ڵ����Ը��������Һ��ɫ����ͼ1��ʾ�ķ�������ʯ�����ѻ���ʵ�飬��ʵ�鰲ȫ�ԵĽǶ���˵����ʵ������д��ڵ���Ҫ������ȱ�ٷ����豸��������̬����ը���¹ʣ�
��5������ʯ�����е�ij�����Ļ�ѧʽ��C18H38���������ѻ���Ӧ���ɹ������A�������ַ����ѻ���Ӧ�����˼������B�������ַ����ѻ���Ӧ�����˶������C��д�������ѻ���Ӧ�Ļ�ѧ����ʽ���л��������û�ѧʽ��ʾ��
��C18H38 $��_{��}^{����}$C10H22+C8H16��
��C10H22 $��_{��}^{����}$C6H14+C4H8��
��C6H14 $��_{��}^{����}$C4H10+C2H4��
���� ��1��ʯ�������ڿ������ϣ�ʯ���͵����������ȵ����Ƭ�������ѻ���Ӧ��
��2�����Ը��������Һ��ɫ����ɫ���Һ���Ϊ���㣬�ϲ���ɫ��״���²���ɫˮ״����˵��ʯ���͵��ѻ������˲��������ͱ��������������������Ը��������Һ�з�����������Ӧ��
��3��������������ˮ�е��巢���ӳɷ�Ӧ�����ɵ��л�������Ϊ��ɫ����״Һ�壬������ˮ���ܶȱ�ˮ������Ϊ��ɫ����״Һ�壬������ˮ���ܶȱ�ˮС��
��4��ʯ�����ѻ����ɵ����徭��ˮ�����������Ը��������Һ����ɫ����˵����ʯ�����ѻ��Ĺ���������̬�����������ɣ���̬����ȼ���ױ���
��5��������ѻ���Ӧ���ֽⷴӦ��Ӧ�û�ѧ��Ӧ�������غ㶨�ɺ���֪������������жϳ���һ�������
��� �⣺��1��ʯ�������ڿ������ϣ�ʯ���͵����������ȵ����Ƭ�������ѻ���Ӧ����ˣ�������������ʯ���͵����壬���Ƭ��������ʯ�����ѻ��Ĵ�����
�ʴ�Ϊ��ʯ���͵����壻������
��2�����Ը��������Һ��ɫ����ɫ���Һ���Ϊ���㣬�ϲ���ɫ����״���²���ɫ��ˮ״����˵��ʯ���͵��ѻ������˲��������ͱ��������������������Ը��������Һ�з�����������Ӧ��
�ʴ�Ϊ��������Ӧ��
��3��������������ˮ�е��巢���ӳɷ�Ӧ�����ɵ��л�������Ϊ��ɫ����״Һ�壬������ˮ���ܶȱ�ˮ������Ϊ��ɫ����״Һ�壬������ˮ���ܶȱ�ˮС�����Կ���Һ��ֳ����㣬�ϲ���²㶼����ɫ����״���м�һ���ˮ״��
�ʴ�Ϊ���ӳɷ�Ӧ��Һ��ֳ����㣬�ϲ���²㶼����ɫ����״���м�һ���ˮ״��
��4��ʯ�����ѻ��Ĺ���������̬�����������ɣ���̬����ȼ���ױ�������ʵ������д��ڵ���Ҫ������ȱ�ٷ����豸��������̬����ը���¹ʣ�
�ʴ�Ϊ��ȱ�ٷ����豸��������̬����ը���¹ʣ�
��5��C18H38�����ѻ���Ӧ���ɹ������A���������غ㶨�ɿ�֪��AΪC8H16������ʽΪC18H38 $��_{��}^{����}$C10H22+C8H16��
�����ַ����ѻ���Ӧ�����˼������B���������غ㶨�ɿ�֪��BΪC4H8������ʽΪC10H22 $��_{��}^{����}$C6H14+C4H8��
�����ַ����ѻ���Ӧ�����˶������C���������غ㶨�ɿ�֪��CΪC2H4������ʽΪC6H14 $��_{��}^{����}$C4H10+C2H4��
�ʴ�Ϊ����C18H38 $��_{��}^{����}$C10H22+C8H16��
��C10H22 $��_{��}^{����}$C6H14+C4H8��
��C6H14 $��_{��}^{����}$C4H10+C2H4
���� ������Ҫ������ʯ�͵����ƣ������л���������ǽ���Ĺؼ����ѶȲ���

 ��У����ϵ�д�
��У����ϵ�д�| A�� | H2��Cl2 | B�� | Cu��Cl2 | C�� | H2��O2 | D�� | Cu��O2 |
| A�� | 1��1 | B�� | 1��4 | C�� | 1��2 | D�� | 4��1 |
| A�� | ���Ӳ��������� | B�� | ԭ�Ӱ뾶������ | ||
| C�� | ��������ϼ���ֵ������ | D�� | �ӹ赽�ȸ��۴�-1��-4 |
| A�� | X����������ϼ�Ϊ6 | B�� | X�ǽ���Ԫ�� | ||
| C�� | Xλ�ڵ�7���ڵڢ�A�� | D�� | XΪ�ǽ���Ԫ�� |
| A�� | �ԣ�A��=0.5 mol/��L•s�� | B�� | �ԣ�B��=0.3 mol/��L•s�� | C�� | �ԣ�C��=12 mol/��L•min�� | D�� | �ԣ�D��=6 mol/��L•min�� |
 ����ѧ�α��н���������ʵ�飺��һ���������״��ͭ˿���ھƾ���������ȣ���ͭ˿�����ں�������������ʢ��Լ2mL�Ҵ����Թ�������������Σ�����ʵ���ж�����ȩ���ɵ�����Ϊͭ˿�ɺڱ�죬�������̼�����ζ
����ѧ�α��н���������ʵ�飺��һ���������״��ͭ˿���ھƾ���������ȣ���ͭ˿�����ں�������������ʢ��Լ2mL�Ҵ����Թ�������������Σ�����ʵ���ж�����ȩ���ɵ�����Ϊͭ˿�ɺڱ�죬�������̼�����ζ
 ��
��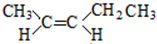 ��
��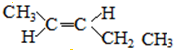 ��
��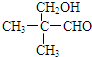 ��
��