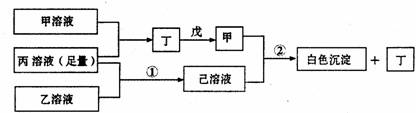
��Ŀ����
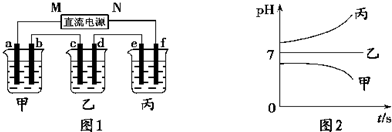
��A��B��C���ֵ������Һ�ֱ�װ�������ձ��У�����ʯī�缫������ͼ��ʾ��ʽ�ڵ�·�����ӡ��պϿ���S��ø�֧·�ĵ���ǿ��I��»I����������I����С��������ȥB����֪����ǿ��IA=IC������ȥC������A��B����Һ���Ⱥ����Ϊ���ȷݣ��������ڵ�·����֪ͨ��A��B�����Һ�ĵ���ǿ������ǰͨ��A��Һ�ĵ���ǿ�ȵ���Դ�С��ϵΪIAB��IA����֪A��B��C�ֱ�ѡ��������Һ��0.1mol��L-1���ᡢ0.1mol��L-1���ᡢ0.1mol��L-1NaCl��Һ��0.1mol��L-1���ᡢ0.1mol��L-1NaOH��Һ��0.1mol��L-1��ˮ����25��ʱ��A��ҺpH<7���ش��������⣺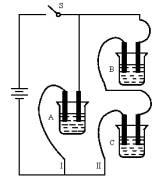
��1��ָ��A��B��C�ǣ�������ǣ�ʲô��Һ��
A________B________C________��
��2������C��Һ�е����̪�Լ��ʺ�ɫ����C��________��
�𰸣�
������
������
| ��1��A������
B����ˮ C��������Ȼ��ƻ��������� ��2��C����������
I��»I����˵��A�ĵ�����B��C�ĵ���ͼ�����ȡ�����B��IA IAB
|

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ