��Ŀ����
�ɶ�����Ԫ����ɵ�X��Y��Z��M����ѧ��ѧ�������������ʣ���������ͼ��ʾ��ת����ϵ�����������ش����⡣

(1)��X��Y��Z����ͬ�ֳ�������Ԫ�أ�M��O2��XΪ____��Z��������һ�������»�������һ�ֲ��д���÷�Ӧ�Ļ�ѧ����ʽ��______________________��
(2)��X��Y��Z����ͬ�ֳ����ǽ���Ԫ�أ�����Z��ʹƷ����Һ��ɫ��
��X�ĵ���ʽΪ_______________��
��Z�����������������Һ��Ӧ�����ӷ���ʽ��______________________��
(3)��X��ˮ��Һ�Լ��ԡ�X��M��Ϊ���壬����ת�����̾���ˮ��Һ�н��С���дһ����������ת������Y��Z��Ӧ�Ļ�ѧ����ʽ��________________��
(2)��X��Y��Z����ͬ�ֳ����ǽ���Ԫ�أ�����Z��ʹƷ����Һ��ɫ��
��X�ĵ���ʽΪ_______________��
��Z�����������������Һ��Ӧ�����ӷ���ʽ��______________________��
(3)��X��ˮ��Һ�Լ��ԡ�X��M��Ϊ���壬����ת�����̾���ˮ��Һ�н��С���дһ����������ת������Y��Z��Ӧ�Ļ�ѧ����ʽ��________________��
(1)Na���ƣ���Na2O2+H2=2NaOH
(2)�� ����SO2+2OH-=SO32-+H2O
����SO2+2OH-=SO32-+H2O
(3)(NH4)2CO3+CO2+H2O=2NH4HCO3[��(NH4)2S+H2S=2NH4HS��(NH4)2SO3+SO2+H2O=2NH4HSO3]
(2)��
 ����SO2+2OH-=SO32-+H2O
����SO2+2OH-=SO32-+H2O(3)(NH4)2CO3+CO2+H2O=2NH4HCO3[��(NH4)2S+H2S=2NH4HS��(NH4)2SO3+SO2+H2O=2NH4HSO3]

��ϰ��ϵ�д�
�����Ŀ
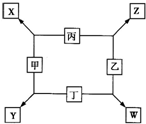 �ס��ҡ������������ɶ�����Ԫ����ɵĵ��ʣ�X��Y��Z��W��Ϊ����������£�XΪ����ɫ���壬Z��������Ϊ����ɫ��W��ʹʪ��ĺ�ɫʯ����ֽ����ɫ����ҵ�ϳ���W����ȡZ����ת����ϵ����ͼ��ʾ��
�ס��ҡ������������ɶ�����Ԫ����ɵĵ��ʣ�X��Y��Z��W��Ϊ����������£�XΪ����ɫ���壬Z��������Ϊ����ɫ��W��ʹʪ��ĺ�ɫʯ����ֽ����ɫ����ҵ�ϳ���W����ȡZ����ת����ϵ����ͼ��ʾ��
 _______��
_______�� ��Ϊ���壬����ת�����̾���ˮ��Һ�н��С���дһ����������ת������Y��Z��Ӧ�Ļ�ѧ����ʽ______________________��
��Ϊ���壬����ת�����̾���ˮ��Һ�н��С���дһ����������ת������Y��Z��Ӧ�Ļ�ѧ����ʽ______________________��