��Ŀ����
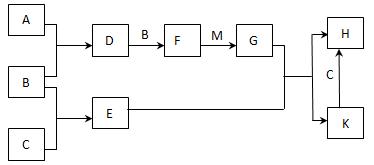
 A-K����ѧ��ѧ�г����ļ������ʣ�����֮���ת����ϵ��ͼ��ʾ����֪������AΪ���嵥�ʣ�BΪ����ɫ��ĩ��G��HΪ��̬���ʣ�I�ڳ�����ΪҺ�壬D��E��F��ˮ��Һ���ʼ��ԣ���C�ı�����Һ��ȡF��E����Ҫ�Ļ����������ش��������⣺
A-K����ѧ��ѧ�г����ļ������ʣ�����֮���ת����ϵ��ͼ��ʾ����֪������AΪ���嵥�ʣ�BΪ����ɫ��ĩ��G��HΪ��̬���ʣ�I�ڳ�����ΪҺ�壬D��E��F��ˮ��Һ���ʼ��ԣ���C�ı�����Һ��ȡF��E����Ҫ�Ļ����������ش��������⣺
��1��д���������ʵĻ�ѧʽ��C______E______
��2��д�����з�Ӧ�Ļ�ѧ����ʽ��
B��D��______
C��E��______
��3������F�ĵ���ʽ��______
��4�������������1L C�ı�����Һ������·��ͨ��0.1mol����ʱ����Һ��pHֵ��������Һ������䣩Ϊ______��
�⣺BΪ����ɫ��ĩ��ӦΪNa202��������AΪ���嵥�ʣ�ӦΪNa����GΪ02��I�ڳ�����ΪҺ�壬ӦΪH2O����FΪNaOH�����뷴Ӧ�ij�ˮ֮���C02����JΪC02��DΪNa2CO3��EΪNaHCO3����C�ı�����Һ��ȡF��E����Ҫ�Ļ�����������CΪNaCl��C����E�ķ�ӦΪ�����Ƽ����Ҫԭ����KӦΪNH3��HΪCl2��
��1�������Ϸ�����֪CΪNaCl��EΪNaHCO3���ʴ�Ϊ��NaCl��NaHCO3��
��2��JΪC02����Na202��Ӧ����Na2CO3��02����Ӧ�ķ���ʽΪ2Na202+2C02=2Na2CO3+02��C����E�ķ�ӦΪ�����Ƽ����Ҫԭ������Ӧ�Ļ�ѧ����ʽΪNaCl+NH3+C02+H2O=NaHCO3��+NH4Cl��
�ʴ�Ϊ��2Na202+2C02=2Na2CO3+02��NaCl+NH3+C02+H2O=NaHCO3��+NH4Cl��
��3��FΪNaOH��Ϊ���ӻ��������ʽΪ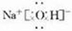 ���ʴ�Ϊ��
���ʴ�Ϊ��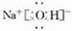 ��
��
��4����ⱥ��ʳ��ˮ�ķ���ʽΪ2NaCl+2H2O Cl2��+H2��+2NaOH������·��ͨ��0.1mol����ʱ������NaOH0.1mol������Һ��pHΪ13���ʴ�Ϊ��13��
Cl2��+H2��+2NaOH������·��ͨ��0.1mol����ʱ������NaOH0.1mol������Һ��pHΪ13���ʴ�Ϊ��13��
������BΪ����ɫ��ĩ��ӦΪNa202��������AΪ���嵥�ʣ�ӦΪNa����GΪ02��I�ڳ�����ΪҺ�壬ӦΪH2O����FΪNaOH�����뷴Ӧ�ij�ˮ֮���C02����JΪC02��DΪNa2CO3��EΪNaHCO3����C�ı�����Һ��ȡF��E����Ҫ�Ļ�����������CΪNaCl��C����E�ķ�ӦΪ�����Ƽ����Ҫԭ����KӦΪNH3��HΪCl2���Դ˽����⣮
������������������ƶ�Ϊ�����ۺϿ������ʵ����ʡ����ԭ���Լ���ѧ�����֪ʶ����Ŀ�Ѷ��еȣ������Թ������Ƶ�����Ϊͻ�ƿڣ�����ʱע����ᣮ
��1�������Ϸ�����֪CΪNaCl��EΪNaHCO3���ʴ�Ϊ��NaCl��NaHCO3��
��2��JΪC02����Na202��Ӧ����Na2CO3��02����Ӧ�ķ���ʽΪ2Na202+2C02=2Na2CO3+02��C����E�ķ�ӦΪ�����Ƽ����Ҫԭ������Ӧ�Ļ�ѧ����ʽΪNaCl+NH3+C02+H2O=NaHCO3��+NH4Cl��
�ʴ�Ϊ��2Na202+2C02=2Na2CO3+02��NaCl+NH3+C02+H2O=NaHCO3��+NH4Cl��
��3��FΪNaOH��Ϊ���ӻ��������ʽΪ
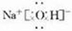 ���ʴ�Ϊ��
���ʴ�Ϊ��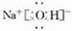 ��
����4����ⱥ��ʳ��ˮ�ķ���ʽΪ2NaCl+2H2O
 Cl2��+H2��+2NaOH������·��ͨ��0.1mol����ʱ������NaOH0.1mol������Һ��pHΪ13���ʴ�Ϊ��13��
Cl2��+H2��+2NaOH������·��ͨ��0.1mol����ʱ������NaOH0.1mol������Һ��pHΪ13���ʴ�Ϊ��13��������BΪ����ɫ��ĩ��ӦΪNa202��������AΪ���嵥�ʣ�ӦΪNa����GΪ02��I�ڳ�����ΪҺ�壬ӦΪH2O����FΪNaOH�����뷴Ӧ�ij�ˮ֮���C02����JΪC02��DΪNa2CO3��EΪNaHCO3����C�ı�����Һ��ȡF��E����Ҫ�Ļ�����������CΪNaCl��C����E�ķ�ӦΪ�����Ƽ����Ҫԭ����KӦΪNH3��HΪCl2���Դ˽����⣮
������������������ƶ�Ϊ�����ۺϿ������ʵ����ʡ����ԭ���Լ���ѧ�����֪ʶ����Ŀ�Ѷ��еȣ������Թ������Ƶ�����Ϊͻ�ƿڣ�����ʱע����ᣮ

��ϰ��ϵ�д�
�����Ŀ