��Ŀ����
��ͼ��A��B��C��D��ͬ���ڻ�ͬ���������Ԫ�أ�
����֪��AԪ�ص���ͼ�Ϊ-3�ۣ�������������ﺬ��56.34%��ԭ�Ӻ�������������������1������AԪ��ԭ��������Ϊ______��ԭ������Ϊ______��AԪ��λ�ڵ�______����______�壮
��д��Ԫ�ط���A��______��B��______��C��______��D��______��
��A��B��C����Ԫ������������ˮ����Ļ�ѧʽ����Ϊ______��______��______������������ǿ����______��
��B��D����Ԫ�غ�����ɵ���̬�⻯��Ļ�ѧʽ����Ϊ______��______����______�ȶ������______��ԭ����ǿ��
����֪��AԪ�ص���ͼ�Ϊ-3�ۣ�������������ﺬ��56.34%��ԭ�Ӻ�������������������1������AԪ��ԭ��������Ϊ______��ԭ������Ϊ______��AԪ��λ�ڵ�______����______�壮
��д��Ԫ�ط���A��______��B��______��C��______��D��______��
��A��B��C����Ԫ������������ˮ����Ļ�ѧʽ����Ϊ______��______��______������������ǿ����______��
��B��D����Ԫ�غ�����ɵ���̬�⻯��Ļ�ѧʽ����Ϊ______��______����______�ȶ������______��ԭ����ǿ��

��1����AԪ�ص���ͼ�Ϊ-3�ۣ������Ϊ+5�ۣ��������������ΪA2O5������56.34%�������ԭ������Ϊx��
��
��100%=56.34%�����x=31��
����������ԭ����������ԭ������Ϊy��ԭ�Ӻ�������������������1����������Ϊy+1��
��y+��y+1��=31�����y=15����AΪPԪ�أ�ԭ�ӽṹ����3�����Ӳ㣬�������5�����ӣ�
Pλ��Ԫ�����ڱ��еĵ������ڵڢ�A�壬
�ʴ�Ϊ��31��15��������A��
��2����Ԫ�������ڱ��е�λ�ÿ�֪��A��B��Cͬ��������Ԫ�أ�B��DΪͬ����Ԫ�أ���BΪS��CΪCl��DΪO��
�ʴ�Ϊ��P��S��Cl��O��
��3��A��B��Cͬ���ڣ�������Ԫ�صķǽ���������ǿ������Ԫ������������ˮ����Ļ�ѧʽ����ΪH3PO4��H2SO4��
HClO4��
Cl�ķǽ�������ǿ����HClO4��������ǿ���ʴ�Ϊ��H3PO4��H2SO4��HClO4��HClO4��
��4��B��Dͬ���壬��̬�⻯��Ļ�ѧʽ����ΪH2S��H2O���ǽ�����O��S�����ȶ���H2O��H2S��H2S�Ļ�ԭ����ǿ��
�ʴ�Ϊ��H2S��H2O��H2O��H2S��
��
| x��2 |
| x��2+16��5 |
����������ԭ����������ԭ������Ϊy��ԭ�Ӻ�������������������1����������Ϊy+1��
��y+��y+1��=31�����y=15����AΪPԪ�أ�ԭ�ӽṹ����3�����Ӳ㣬�������5�����ӣ�
Pλ��Ԫ�����ڱ��еĵ������ڵڢ�A�壬
�ʴ�Ϊ��31��15��������A��
��2����Ԫ�������ڱ��е�λ�ÿ�֪��A��B��Cͬ��������Ԫ�أ�B��DΪͬ����Ԫ�أ���BΪS��CΪCl��DΪO��
�ʴ�Ϊ��P��S��Cl��O��
��3��A��B��Cͬ���ڣ�������Ԫ�صķǽ���������ǿ������Ԫ������������ˮ����Ļ�ѧʽ����ΪH3PO4��H2SO4��
HClO4��
Cl�ķǽ�������ǿ����HClO4��������ǿ���ʴ�Ϊ��H3PO4��H2SO4��HClO4��HClO4��
��4��B��Dͬ���壬��̬�⻯��Ļ�ѧʽ����ΪH2S��H2O���ǽ�����O��S�����ȶ���H2O��H2S��H2S�Ļ�ԭ����ǿ��
�ʴ�Ϊ��H2S��H2O��H2O��H2S��

��ϰ��ϵ�д�
�����Ŀ
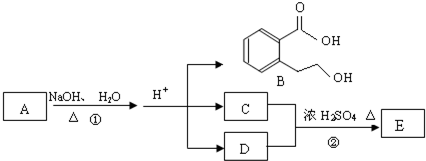




 ����CH2=CH2+H2O
����CH2=CH2+H2O ��ͼ��A��B��C��D��ͬ���ڻ�ͬ���������Ԫ�أ�
��ͼ��A��B��C��D��ͬ���ڻ�ͬ���������Ԫ�أ�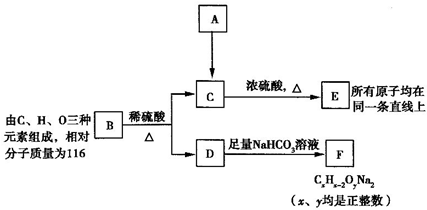
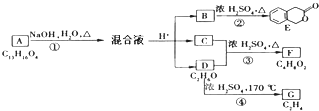
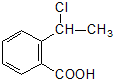 �ϳ�
�ϳ�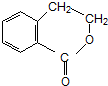 ���÷�Ӧ����ͼ��ʾ����ע����Ӧ������
���÷�Ӧ����ͼ��ʾ����ע����Ӧ������