��Ŀ����
��һ����̼��������443��473K�¶��£�������������ʱ���Է�Ӧ����n��5��8�������������˹��ϳ����͵ķ���֮һ��
(1)д����CnH2n+2��ʾ���˹��ϳ����͵Ļ�ѧ����ʽ����ƽ��________��
(2)������ܱյĺϳ�����ͨ��ǡ������ȫ��Ӧ��CO��H2������ȫ��Ӧ�ϳ������¶Ȳ��䣬����������ѹǿ���͵�ԭ����2/5��ͨ������˵����ʱ�����������ɣ�
�𰸣�
������
������
|
����(1)nCO��(2n��1)H2��CnH2n+2��nH2O ����(2)�������Ϸ���ʽ�ɵó��� |

��ϰ��ϵ�д�
�����Ŀ
(15��)
�ҹ��зḻ����Ȼ����Դ������Ȼ��Ϊԭ�Ϻϳ����ص���Ҫ��������ͼ��ʾ��ͼ��ij
Щת�����輰������δ�г�����
����д���пհף�
��1����֪0.5 mol�����0.5 molˮ������t �棬p k Paʱ����ȫ��Ӧ����һ����̼���������ϳ�������������a kJ�������÷�Ӧ���Ȼ�ѧ����ʽ�ǣ� ______��
��2�����������У���ҵ�Ϸ���H2��CO2�����ķ�����___________��
| A���������ͨ������������Һ��������Һ�м����� |
| B���������ѹ��ȴ��ʹCO2Һ�� |
| C��������ð�ˮϴ�� |
| D���������ͨ�뵽ʯ�ҽ���Ȼ��������չ��壬 |
��4��������������Դ����������߾���Ч�棬����Ҳ�Ƕ���ᡢ��ȫ���ฺ��ı��֣������߶κͼ�ͷ����ͼ�е���������������Դ�����
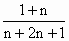 ��
��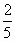 ��n��3�������������ɣ�
��n��3�������������ɣ�
