��Ŀ����
��10�֣��±�ΪԪ�����ڱ���һ���֣���ش��й����⣺
(1) �����ٵ�ԭ�ӽṹʾ��ͼ��______________�� ���Ԫ�����ƣ�____________
(2) �ۢܢ�ԭ�Ӱ뾶�ɴ�С��˳����______________________����Ԫ�ط��ţ�
(3) ��������õĽ�����_________���ǽ�������ǿ��Ԫ����________��(��дԪ�ط���)
(4) �������γ��������������Ԫ����__________��д����Ԫ�ص�����������������������Ӧˮ���ﷴӦ�����ӷ���ʽ��
______________________________________________________________________��
(5) �õ���ʽ��ʾ����Ļ�������γɹ��̣�
______________________________________________________________________��
| | IA | IIA | IIIA | IVA | VA | VIA | VIIA | 0 |
| 2 | | | | | �� | | �� | |
| 3 | | �� | �� | �� | | �� | �� | �� |
| 4 | �� | | | | | | �� | |
(2) �ۢܢ�ԭ�Ӱ뾶�ɴ�С��˳����______________________����Ԫ�ط��ţ�
(3) ��������õĽ�����_________���ǽ�������ǿ��Ԫ����________��(��дԪ�ط���)
(4) �������γ��������������Ԫ����__________��д����Ԫ�ص�����������������������Ӧˮ���ﷴӦ�����ӷ���ʽ��
______________________________________________________________________��
(5) �õ���ʽ��ʾ����Ļ�������γɹ��̣�
______________________________________________________________________��
(1) �� ��2��Mg>Al>Si (3) K F
�� ��2��Mg>Al>Si (3) K F
(4) Al Al(OH)3+ OH��= AlO2��+ 2H2O
(5)
 �� ��2��Mg>Al>Si (3) K F
�� ��2��Mg>Al>Si (3) K F (4) Al Al(OH)3+ OH��= AlO2��+ 2H2O
(5)

��1�����ǵ�Ԫ�أ�ԭ�ӽṹʾ��ͼΪ ������Br���������塣
������Br���������塣
��2��ͬ�����������ң�ԭ�Ӱ뾶��С������Ӧ����Mg>Al>Si��
��3��ͬ�����������ҽ������������ǽ���������ǿ��ͬ�������϶��½���������ǿ���ǽ��������������Խ�������ǿ����K���ǽ�������ǿ��F��
��4��������������������������������Ʒ�Ӧ�ķ���ʽΪAl(OH)3+ OH��= AlO2��+ 2H2O��
��5������ֱ���Mg��S���γɵĻ�ѧ�������Ӽ��������γɹ���Ϊ ��
��
 ������Br���������塣
������Br���������塣��2��ͬ�����������ң�ԭ�Ӱ뾶��С������Ӧ����Mg>Al>Si��
��3��ͬ�����������ҽ������������ǽ���������ǿ��ͬ�������϶��½���������ǿ���ǽ��������������Խ�������ǿ����K���ǽ�������ǿ��F��
��4��������������������������������Ʒ�Ӧ�ķ���ʽΪAl(OH)3+ OH��= AlO2��+ 2H2O��
��5������ֱ���Mg��S���γɵĻ�ѧ�������Ӽ��������γɹ���Ϊ
 ��
��
��ϰ��ϵ�д�
�����Ŀ


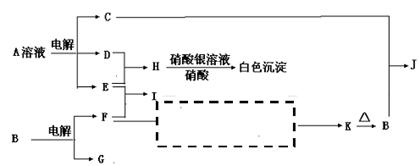

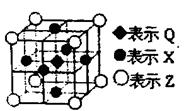
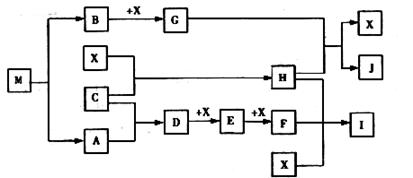
 ��д��D��X��Ӧ�Ļ�ѧ����ʽ
��д��D��X��Ӧ�Ļ�ѧ����ʽ