��Ŀ����
8����1��N2��g��+3H2��g��?2NH3��g����H=-94.4kJ/mol������ʱ����ϵ�и�����Ũ����ʱ��仯��������ͼ��ʾ��a����1L�����з�����Ӧ��ǰ20min��v��NH3��=0.050 mol•��L•min��-1���ų�������Ϊ47.2 kJ��b.25minʱ��ȡ�Ĵ�ʩ�ǽ�NH3�ӷ�Ӧ��ϵ�з����ȥ��c��ʱ��III�����·�Ӧ��ƽ�ⳣ������ʽΪ$\frac{0��{5}^{2}}{0.7{5}^{3}•0.25}$����ͼ�����ݱ�ʾ��������������

��2�����Ṥҵ�Ļ����ǰ��Ĵ��������ڴ����������·������·�Ӧ��
��4NH2��g��+5O2��g��+6H2O��g����H=-905kJ/mol����Ӧ
��4NH2��g��+3O2?2N2��g��+6HO��g����H=-1268kJ/mol����Ӧ
a���ɷ�Ӧ�٢ڿ�֪��ӦN2��g��+O2��g��?2NO��g���ķ�Ӧ�ȡ�H=+181.5 kJ/mol
b����Ӧ����һ���������ܣ���ܡ����ܡ����Է���Ӧ�������ǡ�H��0���ǻ��Ҷ�����ķ�Ӧ��
��3�����ݷ�Ӧ�ڿ�����Ƴ�ֱ�ӹ���ʽ����ȼ�ϵ����ͼ��ʾ����ͼ��AΪ������������������������缫��Ӧ����ʽΪ2NH3-6e-+6OH-=N2+6H2O��
��4��NH3��N2H4�����л�ԭ�ԣ�����������ǿ��������Ӧ��������һ�������£������Ա�˫��ˮ����Ϊ����̬����д���÷�Ӧ�Ļ�ѧ����ʽ2NH3+3H2O2=N2+6H2O��
���� ��1��a����ѧ��Ӧ����v=$\frac{��c}{��t}$���ͼ��������������㼴�ɣ�
b������ͼʾ��25��ʱ������Ũ��Ѹ�ټ�С���ݴ˻ش�
c������ƽ�ⳣ��K���ڸ�������ƽ��Ũ��ϵ���η��ij˻�����Ӧ��ƽ��Ũ��ϵ���η�֮���ı�ֵ���ش�
��2��a��4NH3��g��+5O2?4NO��g��+6H2O��g������H=-905kJ/mol��
4NH3��g��+3O2��g��?2N2��g��+6H2O��g������H=-1268kJ/mol�ڣ�
���ݸ�˹���ɢ�-�ڵõ���2N2��g��+2O2��g��=4NO��g���������Ȼ�ѧ����ʽ��˹���ɼ�������жϣ�
b�����ݷ�Ӧ�Ƿ��Է����оݡ�H-T��S��0���жϷ�Ӧ�Ƿ��Է���
��3����ͼ��֪��A��ͨ���Ϊ����������������Ӧ��Ϊ�����������ڼ��������·ŵ����ɵ�����ˮ��
��4�������Ա�˫��ˮ����Ϊ����̬�������ݵ����غ��ԭ���غ���д��ƽ����ʽ���ɣ�
��� �⣺��1��a����ѧ��Ӧ����v=$\frac{��c}{��t}$=$\frac{1.0mol/L}{20min}$=0.050 mol•��L•min��-1�������Ȼ�ѧ����ʽ�����壬����2mol�����ų�����94.4kJ������ǰ20min������1mol/L�İ������ų���������47.2 kJ���ʴ�Ϊ��0.050 mol•��L•min��-1��47.2 kJ��
b����ͼʾ��25��ʱ������Ũ��Ѹ�ټ�С��25minʱ��ȡ�Ĵ�ʩ�ǣ���NH3�ӷ�Ӧ��ϵ�з����ȥ���ʴ�Ϊ����NH3�ӷ�Ӧ��ϵ�з����ȥ��
c��ƽ�ⳣ��K���ڸ�������ƽ��Ũ��ϵ���η��ij˻�����Ӧ��ƽ��Ũ��ϵ���η�֮���ı�ֵ����Ӧ��ƽ�ⳣ������ʽΪK=$\frac{{c}^{2}��N{H}_{3}��}{c��{N}_{2}��•{c}^{3}��{H}_{2}��}$��ʱ��III�����´�����Ӧ���ݣ���Ӧ��ƽ�ⳣ������ʽΪK=$\frac{0��{5}^{2}}{0.7{5}^{3}•0.25}$���ʴ�Ϊ��$\frac{0��{5}^{2}}{0.7{5}^{3}•0.25}$��
��2��a��4NH3��g��+5O2?4NO��g��+6H2O��g������H=-905kJ/mol��
4NH3��g��+3O2��g��?2N2��g��+6H2O��g������H=-1268kJ/mol�ڣ�
���ݸ�˹���ɢ�-�ڵõ���2N2��g��+2O2��g��=4NO��g����H=+363KJ/mol����������ΪNO���Ȼ�ѧ����ʽΪ��N2��g��+O2��g��?2NO��g������H=+181.5 kJ/mol���ʴ�Ϊ��+181.5 kJ/mol��
b����Ӧ��4NH2��g��+5O2��g��+6H2O��g����H=-905kJ/mol��������H��0���ǻ��Ҷ�����ķ�Ӧ�������κ������£���Ӧ���Է����У��ʴ�Ϊ���ܣ���H��0���ǻ��Ҷ�����ķ�Ӧ��
��3����ͼ��֪��A��ͨ���Ϊ����������������Ӧ��Ϊ�����������ڼ��������·ŵ����ɵ�����ˮ���缫��ӦʽΪ��2NH3-6e-+6OH-=N2+6H2O��
�ʴ�Ϊ��������2NH3-6e-+6OH-=N2+6H2O��
��4������˫��ˮ����Ϊ����̬����ÿmol��ʧ3mol���ӣ�H2O2�õ�������H2O��ÿmol��2mol���ӣ��������Ӻ���������ӻ�ѧ������֮��Ϊ2��3���پ�ԭ���غ���д��ѧ����ʽΪ��2NH3+3H2O2=N2+6H2O���ʴ�Ϊ��2NH3+3H2O2=N2+6H2O��
���� ���⿼�鷴Ӧ�ȵļ��㡢��ѧƽ���������ơ�ԭ��ع���ԭ���ȣ���Ŀ��Ϊ�ۺϣ��ѶȲ���


| A�� | �ܢݢޢ�⑪⑫ | B�� | �ڢۢܢݢޢ� | C�� | �٢ܢݢޢ� | D�� | �٢�⑪⑭��� |
| A�� | ��ҵ������������ˮ�ࡢƯ�ۼ�������ʯұ����������Ҫ��ʯ��ʯΪԭ�� | |
| B�� | δ�����ƻ��������Ʊ���ɫ������ƻ����֭Һ����������Һ������Ӧ | |
| C�� | ����н�ֹ����CaO2����������������������CaO2���ڼ�������������������������л��� | |
| D�� | ����ʵʩ����ȼ�ϡ��������������������Լ������������͵���������Ի�������Ⱦ�� |
| A�� | 4�� | B�� | 8�� | C�� | 12�� | D�� | 24�� |
| A�� | pH=a�İ�ˮ��Һ��ϡ��10������pH=b����a=b+1 | |
| B�� | ����AgCl��AgI���������Һ��c��Ag+����c��Cl-��=c��I-�� | |
| C�� | 0.1mol/LNH4Cl��0.1mol/L��ˮ�������ϣ�pH��7����c��NH3•H2O����c��NH4+����c��Cl-����c��OH-�� | |
| D�� | �������ʵ�����NaHC2O4��Na2C2O4����Һ��2c��Na+��=3[c��HC2O4-��+c��C2O42-��+c��H2C2O4��] |
| A�� | ����ͬ����Ԫ�صĵ�һ�����ܱ仯���ƣ��Ƴ�P�ĵ�һ�����ܱ�S�� | |
| B�� | ��������Ԫ����������ϼ����������Ĺ�ϵ���Ƴ�±��Ԫ����������ϼ۶���+7 | |
| C�� | ������һ�����ڻ�ѧ�� | |
| D�� | ������������м���ֻ����109��28�� |
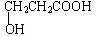 ��������������ˮ����ѡ����ס�����ԭ��������ˮ�����γ�����������ʵ��۵����Ը�����Է���������ӽ������������۵㣬ԭ���Ǹ����ʷ��Ӽ���γ������
��������������ˮ����ѡ����ס�����ԭ��������ˮ�����γ�����������ʵ��۵����Ը�����Է���������ӽ������������۵㣬ԭ���Ǹ����ʷ��Ӽ���γ������