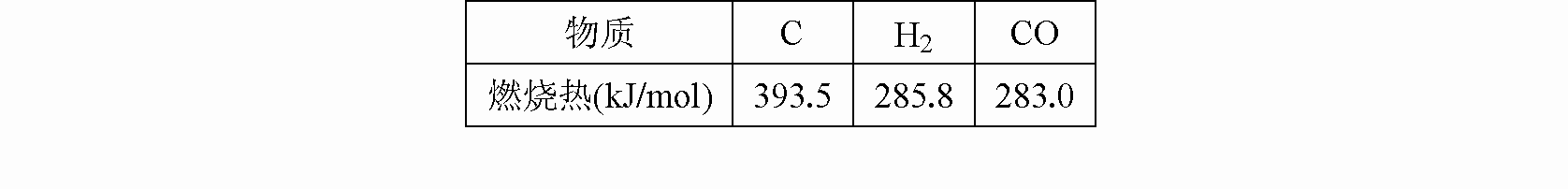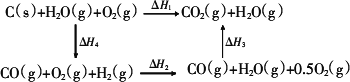��Ŀ����
��֪��
�ٽ�úת��Ϊˮú������Ҫ��ѧ��ӦΪC(s)+H2O(g) CO(g)+H2(g)��
CO(g)+H2(g)��
��C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
C(s) +O2(g)=CO2(g) ��H=-393.5 kJ/mol
CO(g) +l/2O2(g)=CO2(g) ��H= -283.0 kJ/mol
H2(g) +l/2O2(g)=H2O(g) ��H= -242.0 kJ/mol
��ش�
(1)����������Ϣ��д��C(s)��ˮ������Ӧ���Ȼ�ѧ����ʽ��________________________��
(2)�ȽϷ�Ӧ�����ݿ�֪��1 mol CO(g)��1 mol H2(g)��ȫȼ�շų�������֮�ͱ�1 mol C(s)��ȫȼ�շų��� �����ࡣ��ͬѧ�ݴ���Ϊ��úת��Ϊˮú������ʹúȼ�շų����������������ͬѧ���ݸ�˹����������ͼ��ʾ��ѭ��ͼ�����ݴ���Ϊ��úת��Ϊˮú����ȼ�շų���������úֱ��ȼ�շų���������ȡ���
�ٽ�úת��Ϊˮú������Ҫ��ѧ��ӦΪC(s)+H2O(g)
 CO(g)+H2(g)��
CO(g)+H2(g)����C(s)��CO(g)��H2(g)��ȫȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
C(s) +O2(g)=CO2(g) ��H=-393.5 kJ/mol
CO(g) +l/2O2(g)=CO2(g) ��H= -283.0 kJ/mol
H2(g) +l/2O2(g)=H2O(g) ��H= -242.0 kJ/mol
��ش�
(1)����������Ϣ��д��C(s)��ˮ������Ӧ���Ȼ�ѧ����ʽ��________________________��
(2)�ȽϷ�Ӧ�����ݿ�֪��1 mol CO(g)��1 mol H2(g)��ȫȼ�շų�������֮�ͱ�1 mol C(s)��ȫȼ�շų��� �����ࡣ��ͬѧ�ݴ���Ϊ��úת��Ϊˮú������ʹúȼ�շų����������������ͬѧ���ݸ�˹����������ͼ��ʾ��ѭ��ͼ�����ݴ���Ϊ��úת��Ϊˮú����ȼ�շų���������úֱ��ȼ�շų���������ȡ���
(3)
��������ס�����ͬѧ�۵���ȷ����_____���� ���ס����ҡ������жϵ�������_____________��
(3)��úת��Ϊˮú����Ϊȼ�Ϻ�úֱ��ȼ������кܶ��ŵ㣬���о����е������ŵ�____________��
(3)��úת��Ϊˮú����Ϊȼ�Ϻ�úֱ��ȼ������кܶ��ŵ㣬���о����е������ŵ�____________��
(1)C(s) +H2O(g)=CO(g) +H2(g) ��H= +131.5 kJ/mol
(2)�ң���ͬѧ������úת��Ϊˮú��Ҫ������������ ��H1= ��H2+ ��H3���ҡ�H2 >0��
(3)������Ⱦ��ȼ�ճ�֣������䣩
(2)�ң���ͬѧ������úת��Ϊˮú��Ҫ������������ ��H1= ��H2+ ��H3���ҡ�H2 >0��
(3)������Ⱦ��ȼ�ճ�֣������䣩

��ϰ��ϵ�д�
�����Ŀ