��Ŀ����
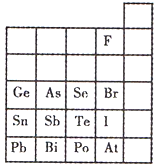
��9�֣�����ͼ��Ԫ�����ڱ��Ķ����ڲ��֣����a��h����8�ֳ���Ԫ�ء�
| | IA[ | O�� | ||||||
| ��һ���� | a | IIA | IIIA | IVA | VA | VIA | VIIA | |
| �ڶ����� | | | | b | c | d | | |
| �������� | e | | f[ | | | g | h | |
��1������gԭ�ӽṹʾ��ͼ________��
��2��д��Ԫ��h�ĵ�����Ԫ��a��c�γɵļ�������û���Ӧ�Ļ�ѧ����ʽ___________________��
��3��д��f������a��d��e����Ԫ���γɵĻ�����ˮ��Һ��Ӧ�Ļ�ѧ����ʽ__________��
��4��a��bԪ���γɵ����������d���ʿ����ȼ�ϵ�أ���KOH��Һ���������Һ����д�������ĵ缫��Ӧʽ��_____________����������______��Ӧ�����������ԭ������OH������____����
��1�� ��1�֣�
��1�֣�
��2��3Cl2��2NH3��N2��6HCl��2�֣�
��3���� 2Al��2NaOH��2H2O��2NaAlO2��3H2����2�֣�
��4��2H2O��O2��4e��4OH����2�֣�������1�֣� ������1�֣�
����

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ


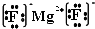
 Ԫ�������ڱ��е�λ�ã���ӳ��Ԫ�ص�ԭ�ӽṹ��Ԫ�ص����ʣ���ͼ��Ԫ�����ڱ���һ���֣�
Ԫ�������ڱ��е�λ�ã���ӳ��Ԫ�ص�ԭ�ӽṹ��Ԫ�ص����ʣ���ͼ��Ԫ�����ڱ���һ���֣�